-
Call Now
1800-102-2727
Van't Hoff Factor: Colligative Properties, Van't Hoff Factor, Formula, Applications of van’t Hoff factor- Abnormal Molar Mass, Practice Problems, FAQs
You know well about the temperature at which water becomes ice, don't you? But what can you tell about the freezing point of say a 4% sodium chloride aqueous solution and of 4% aluminium chloride aqueous solution? Will the freezing point be the same as pure water or higher or less?
I shall give a clue, perhaps. In Europe and other countries, salt (sodium chloride) is sprinkled to remove ice formed on roads. The salt melts away the ice to water to flow off the road. Funny. Ni, it is science and let us know more about this phenomenon.
Table of Content
- Colligative Properties
- van’t Hoff Factor
- Formula of van’t Hoff Factor
- Application of van’t Hoff factor
- Abnormal Molar mass
- Practice Problems
- Frequently Asked Questions(FAQs)
Colligative Properties.
Each pure liquid has unique characteristics, like vapour pressure, melting point, boiling point, osmotic pressure etc,. But when a solute is dissolved to make a solution, the characteristics change a little bit proportional to the concentration of the particles present in the solution. Such dependency of the solution properties on the number of particles present in the solution is referred to as colligative properties.
To put things clear, let us take three solutions, one Molar concentration of glucose, sodium chloride and aluminium sulphate at room temperature in a closed container. The vapour pressure of all these three solutions will be different from that of the pure water vapour pressure. Vapour pressure order will be in the order - pure water> 1M Glucose > 1M NaCl > 1M ammonium sulphate.
Change in the vapour pressure, obviously will change all the related properties like melting point, boiling point and osmotic pressure. Higher the number of particles in the solution, lesser the solvent molecules that can form vapour resulting in lowering of vapour pressure of the solvent in the solution.
Compared to pure water, 1M glucose solution contains additional solute particles,and hence decreases the vapour pressure. In case of sodium chloride and aluminium sulphate, the salts undergo dissociation in water forming more number of particles or ions. Each sodium chloride gives two particles (1 Na and 1 Cl) or ions while aluminium sulphate dissociates to give five ions or particles (2 Al and 3 Sulphate ions). Since the number of particles is higher, aluminium sulphate brings about a larger effect on colligative properties than sodium chloride.
On the other hand acetic acid undergoes association by hydrogen bonds, such that the number of particles will be less than the 1M acetic acid solute. So compared to glucose solution acetic acid will show lesser deviation than glucose solution.
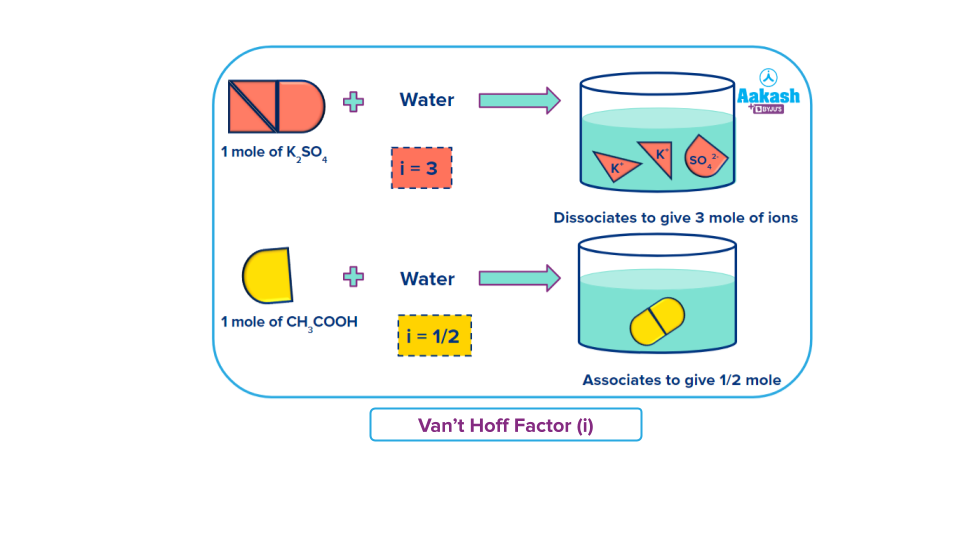
Van’t Hoff Factor
In 1866 ,van’t Hoff Jacobus Henricus(Dutch chemist) suggested a correction factor “i” known as van’t Hoff factor to express the extent of association or dissociation in solution.
The Van't Hoff factor is the ratio of the number of particles in the solution to the particles before dissolution in its natural state irrespective of their charges.
When particles of solute associates in solution van’t Hoff factor is less than 1
When the particles of solute dissociates in solution van’t Hoff factor is greater than 1
When the particles of solute neither associates nor dissociates van’t Hoff factor is equal to 1.
Formula of van’t Hoff Factor
van’t Hoff factor 'i' can be expressed in several ways-
When applied in the colligative properties, the factor can be expressed in practical measurable quantities as-
The colligative properties expression, hence, should have incorporated this van’t Hoff factor, as most of the ionic substances are either dissociated or associated in solution changing the number of particles away from 1.
Colligative properties, hence has to be modified with the correction factor of van’t Hoff factor. The modified relations are-
Relative lowering of vapour pressure
Elevation in boiling point
Depression in freezing point
Osmotic pressure
Where, PAo= vapour pressure of pure solvent
PA= vapour pressure of solvent present in the solution
XB= mole fraction of solute
Tb= elevation in boiling point
Tf= depression in freezing point
= osmotic pressure
m= molality
Kb= molal elevation constant
Kf= molal depression constant
R= universal gas constant
T= Temperature
i= van’t Hoff factor
C= concentration of the solution.
Applications of van’t Hoff factor
(a) Calculation of degree of dissociation of solute particles:
An ⇌ nA
No of moles dissolved 1 mol 0
No of moles after dissociation 1- n
Total number of moles present in the solution = (1-)+n
van’t Hoff factor
Or
(b) Calculation of degree of association of solute particles:
An ⇌ nA
No of moles dissolved 1 mol 0
No of moles after dissociation 1- /n
Total number of moles present in the solution = (1-)+/n
Van’t Hoff factor
Or
Abnormal Molar mass
There are instances when it is discovered that the theoretical values of molar mass, when estimated from the colligative properties of the solutions, differ from the empirically acquired values. Abnormal molar masses are the common term used to describe these readings.
According to Van't Hoff, when solutes dissolve in a solvent, they separate into ions. The breakdown of solute molecules into ions causes an increase in the number of particles, which in turn influences the colligative characteristics because colligative properties are solely dependent on the number of solute particles.
There will be one mole of Na+ ions and one mole of Cl- ions in the resulting solution when one mole of sodium chloride is dissolved in one kilogramme of water. However, we only account for one mole of NaCl when computing the molar mass using colligative characteristics.
Due to hydrogen bonding, ethanoic acid (acetic acid) dimerizes in benzene. This occurs in solvents with low dielectric constant values. Dimerization will result in fewer particles in this scenario.
Practice Problems
Q1. Given that calculated colligative property is 10.09 atm and observed colligative property is 15 atm. What is the degree of dissociation () of calcium chloride?
Given that the calculated and observed colligative properties are 8.09 atm and 13 atm respectively. What is the calcium halides degree of dissociation ()?
Solution:
=0.3
=30%
Q2. The van't Hoff factor used to determine molecular mass if is the degree of dissociation of M2SO4 where M is alkali earth metal.
Solution: M2SO4 ⇌ 2 M++ SO42-
1 0 0
1- 2
Van’t Hoff factor
Q3. In aqueous solution, K2HgI4 has 60% ionisation. The van't Hoff factor value is
Solution: The ionisation of K2HgI4in aqueous solution is as follows:
Where, n=number of ions
= degree of dissociation or association
i= 1+(n-1)
i = 1+0.6(3-1)
i= 2.2
Q4. A NaCl solution in 0.2 m of water with a molal elevation constant of Kb =0.52 K Kg mol-1 will boil at
Solution: Tb=i Kbm
=2✕0.52✕0.2
=0.208
So, (Tb)solution= 100+ 0.208=100.208o C
Frequently Asked Questions (FAQs)
Q1. Describe how dissociation and association differ.
Answer:
|
Dissociation |
Association |
|
The observed molar mass is below the expected value. |
The measured molar mass is higher than the expected value. |
|
The van't Hoff factor is never less than one. |
The van't Hoff factor is never greater than one. |
|
The colligative property values are higher than anticipated. |
The colligative property values are lower than anticipated. |
Q2. What is the van't Hoff factor for aqueous solution of calomel (Hg2Cl2)?
Answer: Calomel formula is Hg2Cl2.
Hg2Cl2 ⇌ Hg22++2Cl-
So van’t Hoff factor is i=1+2 =3.
Q3.Why, in the case of some anomalous solutes, is the molar mass estimated by measuring the colligative property?
Answer: Due to dissociation or association, the number of particles changes. The molar mass changes because the colligative characteristics are solely dependent on the quantity of solute particles.
Q4. For a diluted aqueous solution of the potent electrolyte barium hydroxide ( Ba(OH)2), the van't Hoff factor is
Answer: Barium hydroxide formula is Ba(OH)2
Ba(OH)2 ⇌ Ba2++2OH-
So van’t Hoff factor i=1+2=3.


