-
Call Now
1800-102-2727
Ferric Chloride-Introduction, Synthesis, Physical Properties, Chemical Properties, Uses, Practice Problems, FAQs
Have you visited some industrial zones, where there are lots of industries that release chemicals in the water as waste products? Have you ever thought about how we can treat such water pollutants?
Especially for sewage treatment, ferric chloride is widely employed in water treatment facilities. In light of sustainable ecological growth, this molecule is crucial.
More than 80% of all ferric chloride is marketed for use in municipal applications, with 37% going toward the treatment of potable water and 53% going toward municipal wastewater. As a result, it can significantly contribute to the provision of highly effective and affordable water-treatment technologies.
FeCl3, which has several applications in industry, medicine, and laboratories. FeCl3 is often used in the syntheses of organic compounds in the lab. Ferric chloride is a Lewis acid that is frequently used in laboratories to catalyze reactions like the Friedel-Crafts reaction of aromatic compounds and the halogenation (chlorination) of aromatic compounds.
Therefore, understanding this critical element, its key compounds, characteristics, and applications is crucial.

TABLE OF CONTENTS:
- What is Ferric Chloride?
- Synthesis of ferric chloride
- Physical properties of ferric chloride
- Chemical properties of ferric chloride
- Uses of Ferric Chloride
- Practice Problems
- Frequently asked questions-FAQs
What is Ferric Chloride (FeCl3) ?
- The inorganic compound ferric chloride is made up of an iron atom with an oxidation state of +3.
- Molysite, also known as ferric chloride, is an orange to brown-black solid that appears in nature. Ferric chloride has a chemical composition FeCl3 and a molecular weight of 162.204 g mol-1respectively.
- In general, ferric chloride is only marginally soluble in water.
- Anhydrous ferric chloride often ranges in colour from orange to brown-black. But it also relies on the perspective from which it is seen. Dark green crystals are light-reflecting, while purple-red crystals are light-transmitting.
- Human tissues as well as other metals are corroded by ferric chloride solution. Using the non-combustible ferric chloride solution, sewage is treated and water is purified.
Synthesis of ferric chloride:
- The reaction of iron and chlorine: Ferric chloride is formed when chlorine molecules are combined with iron. Anhydrous ferric chloride is primarily prepared using this approach.
- Reaction of iron ore with HCl: From iron ore, ferric chloride aqueous solutions can also be produced. In the presence of strong hydrochloric acid, iron ore from ferric chloride solution.
- Reaction of ferrous chloride with chlorine: A ferric chloride solution can be formed from ferrous chloride that is in the +2 oxidation state. Solution of ferric chloride with a +3 oxidation state is obtained by the interaction of ferrous chloride with chlorine molecules.
- Reaction of ferrous chloride with oxygen: In the presence of hydrochloric acid, ferrous chloride interacts with oxygen to form ferric chloride solution.
Physical properties of ferric chloride:
The physical properties of ferric chloride are as follows:
- The anhydrous form of ferric chloride has a crystalline structure with a melting point of 306.6 °C. The colour of anhydrous ferric chloride is generally orange to brown-black colour. Anhydrous ferric chloride consists of octahedral centres that are interconnected with the help of 2 coordinate chloride ligands. Ferric chloride has a boiling point of 315 °C.
- Unlike anhydrous forms of ferric chloride, aqueous solutions generally appear colourless or yellow.
- Highly soluble in both methanol and diethyl ether. It is not soluble in ethyl acetate. Heat is emitted when dissolved in water, indicating that the reaction is exothermic.
- The dissolution of ferric chloride in water produces an acidic solution
- Molar weight - 162.204 g mol-1 for anhydrous ferric chloride. 270.295g mol-1 for hexahydrate ferric chloride.
- Color - Orange to brown-black for anhydrous forms. Aqueous solutions of ferric chloride are generally colourless or yellow.
- Odour - Faint HCl.
- Density of anhydrous ferric chloride comes out to be 2.90g cm-3 whereas the density of aqueous ferric chloride solution is 1.82g cm-3
Chemical reactions of ferric chloride:
- Anhydrous ferric chloride is a deliquescent dark crimson solid. At around 300°C, it sublimes, and its vapour density corresponds to the dimeric formula Fe2Cl6. The dimer separates in FeCl3 which is formed at high temperatures. The dissociation into FeCl3 is a slow process. Complete at 750૦C. Above this temperature, it decomposes into ferrous chloride as well as chlorine.
![]()
- Because it is soluble in non-polar solvents such as ether, alcohol, and others, anhydrous ferric chloride acts as a covalent molecule. The chlorine bridge structure is represented in the below image.
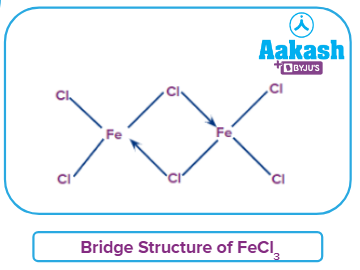
- It is water-soluble. Because of its hydrolysis, the solution is acidic in nature. The reaction below shows this.
To prevent hydrolysis, the solution is stabilized by adding hydrochloric acid.
- Ammonia is absorbed by anhydrous ferric chloride.
- Ferric chloride is a strong oxidizer.It converts stannous chloride to stannic chloride by oxidation.
It converts SO2 into H2SO4 via oxidation.
Uses of ferric chloride:
Ferric chloride finds various applications in pharmaceutical and industrial sectors as well. The various applications of ferric chloride are as follows:
- Ferric chloride is used in the treatment of sewage and the purification of water.
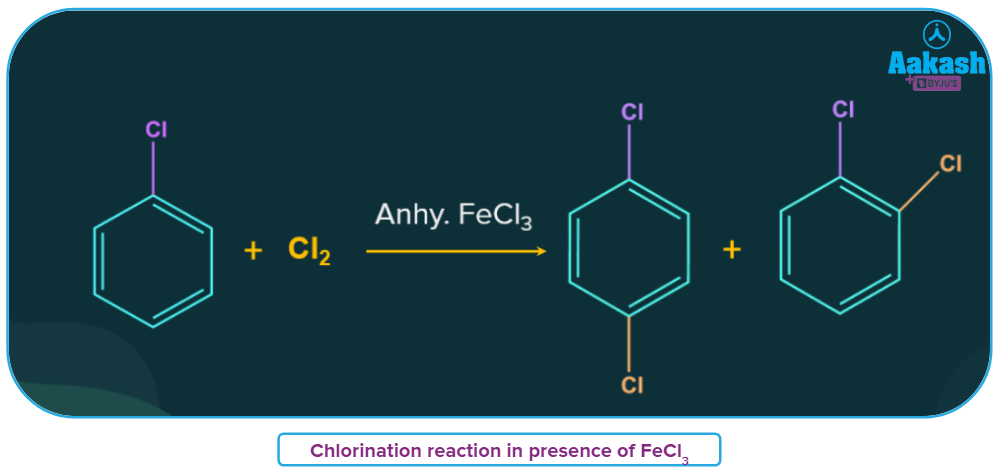
- Catalysts like ferric chloride are frequently utilized in organic reactions. Major examples include Friedel crafts alkylation/acylation and chlorination of aromatic compounds.
- Ferric chloride is used in the colourimetric analysis of phenols.
- Ferric chloride is additionally utilized in veterinary practice to treat animal claws overcropping, which leads to bleeding.
Practice Problems:
Q1. With potassium ferrocyanide, ferric chloride generates a ___________ complex
A. Dark red
B. Prussian blue
C. Dark brown
D. White colour
Answer: B)
Solution: With potassium ferrocyanide (K4[Fe(CN)6]), ferric chloride generates a Prussian blue complex called Ferri ferrocyanide. Below is the reaction to that.
Q2. __________ is used to dehydrate ferric chloride.
A. Potassium ferrocyanide.
B. Stannic chloride.
C. Thionyl chloride.
D. Ammonium hydroxide
Answer: C)
Solution: Thionyl chloride is used to dehydrate ferric chloride. Heat the hydrated ferric chloride with thionyl chloride to dehydrate it in FeCl3 . Below is the required reaction for that.
Q3. Which of the following acids, when combined with ferric chloride solution, will produce purple colour?
A. - Hydroxypropanoic acid
B. Acetic acid
C. Glycolic acid
D. Salicylic acid
Answer: D)
Solution: Depending on the type of enol group involved, neutral ferric chloride reacting with it produces distinctive colours including violet, blue, and green. Alcohols and phenols react differently with neutral ferric chloride, whereas phenols react to produce various colours, hence this reaction is employed as a test to distinguish between the two. Salicylic acid is the one which is a phenolic compound.
Q4. What substance is frequently referred to as molysite?
A. FeO
B. FeCl2
C. FeCl3
D. FeSO4
Answer: C)
Molysite, The rare mineral, which is typically associated with fumaroles of the volcanic and other types, is the natural equivalent of FeCl3. Molysite, also known as ferric chloride, is an orange to brown-black solid that appears in nature. Ferric chloride has a chemical composition FeCl3 and a molecular weight of 162.204 g mol-1respectively.
Frequently asked questions- FAQ
Q1. How many HCl molecules are produced when one hydrated ferric chloride molecule is heated?
Answer: On heating hydrated ferric chloride anhydrous FeCl3 is not formed but it is changed to Fe2O3
FeCl3 gets hydrolysed by the water of crystallization on heating. Since, 6 molecules HCl are produced when two hydrated ferric chloride molecules are heated, then for one molecule, 3HCl molecules are produced.
Q2. What is the oxidation number of iron in the famous compound ferric chloride?
Answer: The formula of ferric chloride comes out to be FeCl3. In this compound chlorine is in -1 oxidation state, now if we assume the oxidation state of iron to be ‘X ’, hence,
Hence, the oxidation number of iron in famous compound ferric chloride is +3.
Q3. Is it okay to touch ferric chloride?
Answer: Although it is very irritating to the skin and eyes, it is only mildly harmful when consumed. Skin contact should be avoided because it is mildly corrosive.
Q4. How do you remove ferric chloride stains?
Answer: We can try with a citric acid solution to remove glass wares stains of Ferric Chloride.


