-
Call Now
1800-102-2727
Henry’s Law - Formula, Statement, Examples, Factors Affecting Henry’s Law Constant and Limitations
Have you ever noticed how a soft drink fizzes when it is opened?
Do you know what makes the soft drink fizz?
Kudos to you if you know the reason! If not, let’s get to understand.
Above the drink, the gas is almost pure carbon dioxide at a pressure slightly higher than atmospheric pressure, before the bottle or can is opened. Dissolved carbon dioxide is present in the drink. When the bottle or can is opened, some of the gas escapes, producing the distinctive hiss. Because the pressure above the liquid has dropped, some of the dissolved carbon dioxide escapes as bubbles. If a glass of the drink is left out in the open, the concentration of carbon dioxide in the soft drink will attain equilibrium with the concentration of carbon dioxide in the air, causing the drink to flatten i.e. it loses its fizzy taste. Therefore, as the pressure above the drink decreases, the solubility of carbon dioxide in the soft drink decreases.

TABLE OF CONTENTS
- Henry’s Law
- Graphical Analysis of Henry’s law
- Examples of Henry’s law
- Factors Affecting Henry’s Law Constant
- Characteristics of Henry’s Law Constant (KH)
- Limitations of Henry’s Law
- Practice Problems
- Frequently Asked Questions - FAQ
Henry’s law
It states that “the partial pressure of the gas in the vapour phase (p) is directly proportional to the mole fraction of the gas (x) in the solution” and is expressed as

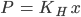
Where KH Henry’s law constant
The practical description for Henry's law is that the solubility of a gas in a liquid is proportional to the partial pressure of that gas present above the liquid.

Above is an illustration of the relationship between a gas's solubility in a liquid and its partial pressure in the atmosphere above the liquid (as dictated by Henry's law). It is important to note that the higher the partial pressure of the gas, the greater its solubility in the liquid.
Graphical Analysis of Henry’s law
The Henry’s law expression is given as follows

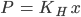
Comparing it with the equation of a straight line, y = mx + C,
Intercept (C) = 0
Slope (m) = KH

Examples of Henry's Law
1. An important application of Henry's Law is in respiratory physiology. It is used to predict how gas dissipates during gas exchange in the alveoli and bloodstream. The amount of oxygen dissolved in the bloodstream is proportional to the partial pressure of oxygen present in the air of the alveoli. Oxygen partial pressure is greater in alveolar air than in deoxidized blood, so oxygen is more likely to dissolve in deoxidized blood. On the contrary, in the case of carbon dioxide, the opposite is true. Since the partial pressure of deoxidized blood is greater than the partial pressure of air in the alveoli, carbon dioxide diffuses out of the solution and returns to a gaseous state. We know that carbon dioxide is much more soluble in plasma than oxygen (about 22 times) and that more carbon dioxide molecules can diffuse through the small pressure gradients of the capillaries and alveoli increase.
2. As the scuba diver descends deeper and deeper into the water, the pressure in the bloodstream increases. When a diver inhales air under high pressure, the bloodstream absorbs gaseous particles such as nitrogen. According to Henry's Law, as pressure increases, so does the solubility of nitrogen in the diver's blood. This prevents nitrogen from escaping from the compressed air in the bloodstream until it escapes by exhalation at low pressure. However, because the diver is in a high pressure environment, nitrogen can only leave the body when he or she reaches lower pressure. Ideally, this should be done as the diver gradually rises to the surface of the water. Unfortunately, divers can rise too fast and nitrogen bubbles can form rapidly. It blocks capillaries and causes a condition called curvature. This is painful and life-threatening. To avoid twisting and the toxic effects of high levels of nitrogen in the blood, the cylinders used by scuba divers are filled with air diluted with helium (11.7% helium, 56.2% nitrogen, 32.1% oxygen).
Factors Affecting Henry’s Law Constant
1. Temperature and pressure - Temperature and pressure are the basic driving forces for the solubility of a gas in a liquid. Increasing the temperature and partial pressure of the gas in the liquid will significantly increase the solubility of the gas. Therefore, the value of Henry's law is constantly changing with changes in gas temperature and partial pressure.
2. Nature of the gas: The nature of the gas dissolved in the liquid plays an important role in determining the value of Henry's constant. All gases have different Henry constants.
3. Solvent type: The type of solvent used to dissolve the gas also affects Henry's constants.
Characteristics of Henry’s Law Constant (KH)
- According to the relation
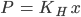 , It has the same units as that of the pressure because there are no units for mole fraction.
, It has the same units as that of the pressure because there are no units for mole fraction. - The different gas-liquid systems have different values of KH.
- KH increases with temperature.
- Its value depends on the intermolecular forces in gas-liquid systems.
- KH decreases with an increase in solubility for the same partial pressure.
Limitations of Henry’s Law
Henry's law applies only under the following conditions
- The gas pressure is not too high.
- The temperature should neither be too low nor too high.
- Gases must not chemically react with the solvent.
- The gas should not dissociate or associate in the solvent.
Practice problems
Q1. H2S is a toxic gas used in qualitative analysis. If the solubility of H2S in water at STP is 0.195m, what is the value of KH?
Solution:
Number of moles of H2S = 0.195
Number of moles of ![]()
Mole fraction of ![]()
Pressure at STP = 0.987 bar
According to Henry’s law, ![]()

Q2. KH for molality of methane in benzene at 298 K is 4.27 × 105. The mole fraction of methane in benzene at 298 K under 760 mm of Hg is
Solution: We know that, ![]()
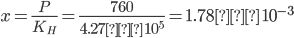
Q3. Henry's law constant for CO2 dissolved in water is 1.67 × 108 at 298 K. Find the mole fraction of CO2 in 1L of soda water when packed under 2.5 atm pressure at 298 K. 
Solution:
Given: 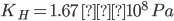
P = 2.5 atm
1 atm = 101325 Pa
Therefore, 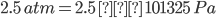
We know that, ![]()
Substituting the values of P and KH,
2.5 × 101325 = 1.67 × 108 × x
x = 1.517 × 10-3
Q4. KH for the solubility of N2 gas in water at 298 K is 1.0 × 105 atm. 0.8 is the mole fraction of N2 in the air. Calculate the moles of N2 from the air dissolved in 10 moles of water at 298 K and 5 atm.
Solution:
Ptotal = 5 atm

YN2 = mole fraction of N2 in air
PN2 = 0.8 × 5 = 4 atm
From Henry’s Law,
![]()

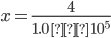
x = 4 × 10-5
1 mol of water contains 4 × 10-5 moles of N2
Therefore, 10 mol of water contains 4 × 10-5 moles of N2
Frequently Asked Questions - FAQ
Question 1. What is the effect of KH on the solubility of a gas in liquid?
Answer: At constant temperature, the solubility of gas (S) varies inversely with Henry’s law constant.
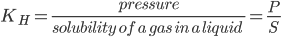
Thus, higher value of Henry’s law constant (KH), the lower is the solubility of a gas in the liquid.
Question 2.Why Henry’s law is not applicable to CO2 and NH3?
Answer: This is due to the fact that these gases react with water.
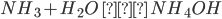
CO2 + H2O ---> H2CO3
They have higher solubilities than expected from Henry’s law due to the reaction of gases such as CO2 and NH3.
Question 3. What are bends?
Answer: Painful effects during the decompression of dissolved air in the blood of scuba divers are known as bends. In order to avoid bends and toxic effects of high concentration of nitrogen in the blood the tanks used by scuba divers are filled with air diluted with helium (the composition of the gases in the tanks of scuba drivers are 11.7% helium, 56.2% nitrogen and 32.1% oxygen).
Question 4.Why is the partial pressure proportional to the mole fraction in Henry’s law?
Answer: Henry’s law is a limiting law that only applies to dilute solutions. The more the system diverges from the ideal behaviour, the range of concentration in which it applies becomes narrower.In dilute solutions, the concentration of solute is approximately proportional to its mole fraction. This is a conceptual conclusion as the steeper the concentration gradient the rate of diffusion will be greater. Here, the concentration of the solute is represented by the partial pressure of gas in the gaseous state and its corresponding mole fraction in the solution state.
Related Topics
|
Depression in Freezing Point |
Elevation in Boiling Point |
|
Relative Lowering of Vapour Pressure |
Osmosis and Osmotic Pressure |
|
Solubility |
Types of Solution |
|
Raoult's Law |
Vapour Pressure |


