-
Call Now
1800-102-2727
Uses of Nitric Acid - Structure, Ostwald Process, Important Properties, Uses, Practice Problems and FAQ
Besides ammonia, nitric acid would undoubtedly be blamed for the prolonged and extended duration of the First World War!
Wonder how? Let’s understand the real narrative behind this!
The Ostwald method for manufacturing nitric acid from oxidation of ammonia, was found just in time for the First World War, and it had a significant role in the war's prolonged duration. This is because Germany formerly lacked nitrate resources to produce nitric acid, which was required for the creation of explosives such as TNT and nitroglycerine used in artillery shells. Some argue that WW1 might not have happened at all if this chemical process in particular, had not occurred.

In fact, most of the nitrates could only be obtained from guano, which is made up of the droppings of fish-eating marine birds and is abundant on the Peruvian islands. When the war broke out, the Atlantic shipping lines to Germany were cut off, and Germany needed a new way to generate nitric acid.
German chemist, Fritz Haber, had discovered a mechanism to convert inert nitrogen gas from air into ammonia just before WW1 broke out. By 1913, Germany produced 30 metric tonnes of ammonia per day. Without a doubt, the Haber-Bosch process for converting N2 to NH3 and the Ostwald process for converting NH3 to nitric acid allowed Germany to continue producing explosives and prolong the war for many years.
In this concept page, we will get to know the important uses of nitric acid in detail.
TABLE OF CONTENTS
- Nitric Acid - Introduction & Important Facts
- Nitric Acid - Structure
- Ostwald Process
- Nitric Acid - Important Properties
- Nitric Acid - Uses
- Practice Problems
- Frequently Asked Questions - FAQ
Nitric Acid - Introduction & Important Facts
Nitric acid (HNO3) is an oxoacid of nitrogen and is certainly a very strong mineral acid. It serves as both a protic solvent and a reagent. It’s conjugate base is a nitrate ion (NO3-). It has a stifling odour and is colourless.
In 1784, an English scientist and physicist named Henry Cavendish was the first to identify the chemical nature and composition of nitric acid.
- Engraver's acid, azotic acid, the spirit of nitre, and aqua fortis are some of the other names for it.
- In its purest state, it is colourless, but as it ages, it takes on a yellow hue. This colour is created when nitric acid is broken down into nitrogen oxides and water. The acid decomposes when exposed to light, releasing nitrogen dioxide (NO2), a brownish gas. The yellowish colour of nitric acid is caused by the presence of small amounts of nitrogen dioxide.
- It is incredibly toxic and caustic. It results in severe skin burns. When it comes in contact with hydroxides, metals, and oxides, it creates nitrate salts.
- Nitric acid is one of the most potent oxidizers known, capable of oxidising almost any metal except gold and platinum.
- Nitric acid is produced through catalytic oxidation of ammonia. It is a common laboratory reagent as well as a key ingredient in explosives and fertiliser manufacture. Nitric acid has a pH of roughly 3.01
Nitric Acid - Structure
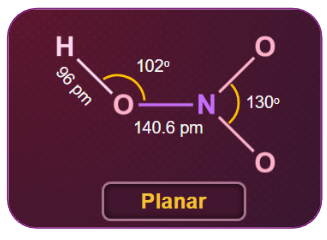
Nitric acid has a planar structure or a chemical structure that is flat. Nitric acid is found in two different resonance forms. It is considered to be in resonance form when there are multiple ways to sketch a compound's Lewis structure. In a compound, a Lewis structure displays the bonding of atoms and the presence of non-bonded pairs of electrons.
Also, one oxygen atom has a charge of -1 and is singly linked to the central nitrogen atom. The nitrogen atom at the core of the molecule has a charge of +1 because it is involved in four covalent bonds (with three oxygen atoms). As a result, the molecule has a net charge of 0 (the positive charge on the nitrogen atom cancels out the negative charge on the oxygen atom).
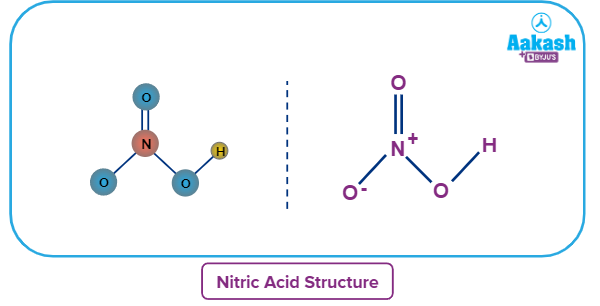
In a gaseous state, it exists in a planar structure.
Ostwald Process
Large-scale industrial production of nitric acid is done by the Ostwald process. Ammonia is oxidised over a platinum catalyst or a platinum-rhodium combination in the Ostwald process. Catalytic oxidation of NH3 in the presence of atmospheric oxygen takes place in this process.
Step 1:
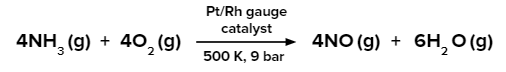
Step 2: The nitric oxide obtained combines with oxygen to give NO2.
Step 3: The nitrogen dioxide then obtained is dissolved in water to give HNO3. The nitric oxide (NO) obtained as a by-product is recycled.
Step 4: Upon distillation, aqueous, HNO3 can be concentrated up to ~68% by mass.
Step 5: It can be further concentrated with concentrated H2SO4 to up to 98% by mass due to dehydration.
![]()
Nitric Acid - Important Properties
- Nitric acid is miscible with water. It also decomposes in ethyl alcohol and reacts violently with most of the organic solvents.
- The molar mass of nitric acid is 63.01 g mol-1
- Nitric acid is a poisonous, corrosive, and suffocating liquid. Nitric acid is available in a variety of concentrations and can be colourless, yellow, or red.
- Nitric acid decomposes into NO2 and O2 when mixed with water, resulting in a brownish-yellow solution. It totally ionises into nitrate and hydronium ions in water.
- Nitric acid is a strong monoprotic acid. It forms solid hydrates such as monohydrate (HNO3.H2O) and trihydrate (HNO3.3H2O) .
- Standing nitric acid decomposes into brown nitrogen dioxide. This is why, despite the fact that fresh nitric acid is colourless, it becomes dark with time.
- Nitric acid behaves as a strong acid, releasing hydronium and nitrate ions.
- It's a powerful oxidant that reacts with a wide range of non-metallic compounds.
- It also reacts with metals, causing them to dissolve and generate metal oxides, among other things.
Example:
- At ambient temperature, 100% pure anhydrous nitric acid is a liquid, but below -41°C, it becomes a colourless white solid that boils at 83°C.
- What we call 'concentrated nitric acid' is actually a 16 M solution of 68% by weight HNO3 in water, which is pale yellow due to photochemical breakdown, which releases NO2.
- When more NO2 is dissolved in the pure material, red 'fuming' nitric acid is produced, which is an exceptionally powerful acid and oxidising agent used in the semiconductor industry to clean silicon wafers. Free Cl2 and nitrosyl chloride can be found in the acid aqua regia (about 3 vols HCl to 1 vol HNO3) (NOCl).
- Aqua regia is capable of attacking even inert metals such as gold and platinum due to the ability of Cl- to stabilise the complexes AuCl4- and PtCl62-.
Nitric Acid - Uses
Nitric acid in Fertilisers
- Nitric acid is used in fertiliser production to make various nitrogenous fertilisers such as calcium nitrate, ammonium nitrate, and others. Nitric acid is a major component that is also produced as a by-product of ammonia production.
- The manufacturing of fertiliser is the principal industrial use of nitric acid. Nitric acid, when mixed with ammonia, forms ammonium nitrate, a wonderful fertiliser. Calcium nitrate is another man-made fertiliser. These fertilisers are utilised on farms all around the United States.

Industrial Use of Nitric Acid
- Nitric acid is a chemical that serves as a starting point for the creation of a variety of other chemicals.
- In the aerospace sector, nitric acid is extensively employed as a rocket propellant.
- It is also used to make nitrogen-based chemicals such as nylon, as well as explosives such as trinitrotoluene (TNT) and nitroglycerin that are military grade and used for national security purposes ideally.
- It is also used in making nitrate salts, dyes, coal tar products, and pharmaceuticals.
- It is also primarily used to purify precious metals such as platinum, gold, and silver.
- In food packaging and paperboard, nitric acid is a frequent component of an adhesive.
Polymer Synthesis using Nitric Acid
- It is used to make a variety of polymers, including polyamides and polyurethanes.
- Many chemical compounds require nitric acid as a starting ingredient. Polymers such as polyamines and polyurethanes are two examples of chemicals that rely heavily on nitric acid for their production. Certain polymerisation reactions can only happen in the presence of nitric acid.
Nitric Acid as an Oxidant
- Because nitric acid is a powerful oxidising agent, it is utilised as an oxidant.
- The oxidation of KA oil (ketone-alcohol oil), which is a combination of cyclohexanone and cyclohexanol, by nitric acid produces adipic acid, which is a precursor to the polymer nylon. The role of nitric acid in this process is to oxidise the oil and produce adipic acid.
Purification of Metals
- Noble metals such as platinum, gold, and silver can be purified and cleaned with nitric acid. When mixed with hydrochloric acid, it produces aqua regia, which can dissolve gold and platinum. Jewellers are usually the ones who purify noble metals.
- Aqua regia is a yellow fuming liquid generated by diluting pure nitric and hydrochloric acids in a 1:3 ratio. It can dissolve noble metals like gold and platinum, hence it is utilised in gold and platinum purification as well as jewellery production.
Use in Daily Life
- Nitric acid's most prevalent application is in schools, where it is frequently used as a laboratory reagent.
- In the woodworking industry, diluted nitric acid is used to create maple and pine wood and give it an aged appearance.
- It is also used in the food industry to clean food and equipment, among other things.
- In food packaging and paperboard, nitric acid is a frequent component of an adhesive.
- When nitric acid and alcohol are combined, they can be used to etch designs on metals such as copper, bronze, and brass. Typically, these are utilised in house décor.

Nitric Acid as Laboratory Reagent
- For laboratory activities, nitric acid is a necessary chemical. It is a pH buffer, cleaning agent, and preservative for water samples that need to be tested for metals.
- Dilute nitric acid (0.5–5.0 %) is employed as a matrix compound for detecting metal traces in solutions in elemental analysis by ICP-MS (Inductively Coupled Plasma Mass Spectrometry), ICP-AES (Inductively Coupled Plasma Atomic Emission Spectrometry), GFAA (Graphite Furnace Atomic Absorption Spectroscopy), and Flame AA (Flame Atomic Absorption Spectrometry).
- Because small concentrations of metal ions could alter the analysis' result, ultrapure trace metal grade acid is required for this assessment.
- Nitric acid is employed in electrochemistry as a chemical doping agent for organic semiconductors and in the purification of raw carbon nanotubes.
- HNO3 is used to detect Cl-(chloride ion), by mixing a sample of AgNO3 solution and nitric acid to see the formation of silver chloride (as white precipitate).
Nitric Acid in Pharmaceutical Industry and Medicine
- Nitric acid is used for drug detection. It is used to spot test alkaloids such as LSD (Lysergic acid Diethylamide). Colourimetric tests, which need nitric acid, are also used to distinguish between morphine and heroin.
- Through a process known as potentization, nitric acid is utilised to make homoeopathic medicines. There are medications available to treat throat sores and tonsillitis, as well as mouth ulcers, piles, and skin problems.
- It can treat warts and boils. When diluted, it can be used to treat indigestion in some specific ways.
- HNO3 in low concentration is also used to treat Dyspepsia.
Nitric Acid as Rocket Propellant
- In the aerospace sector, nitric acid is utilised as a rocket propellant. The red fuming nitric acid, which is a storable oxidant, is the name for this type of nitric acid.
- It is composed of 84% nitric acid, 13% dinitrogen tetroxide, and 1 to 2% water.
- In liquid-fueled rockets, nitric acid has been utilised as an oxidizer in various forms such as red fuming nitric acid and white fuming nitric acid. The BOMARC (Boeing Michigan Aeronautical Research Centre) missile used IRFNA (Inhibited Red Fuming Nitric Acid) as one of three liquid fuel components.

Nitric Acid as Precursor to Organic Nitro-Compounds
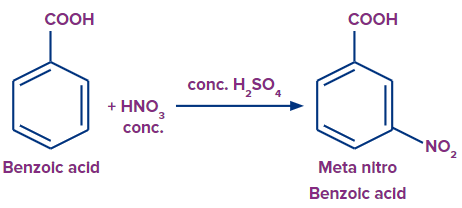
- The nitro group is a versatile functional group in organic synthesis, both industrial and non-industrial. Electrophilic aromatic substitution puts a nitro substituent onto various aromatic compounds using a mixture of nitric and sulfuric acids.
- For example, TNT is produced by the nitration of toluene.
Practice Problems
Q. 1. Silver nitrate is formed when silver reacts with
a. Hydrochloric acid
b. Nitric acid
c. Hydrogen sulphate
d. Caro’s acid
Answer: Silver nitrate contains a NO3- ion which is obtained from nitric acid when Ag reacts with
So, option B) is the correct answer.
Q. 2. The product obtained when nitric acid reacts with
a. H2S
b. Colloidal Sulphur
c. Benzoic Acid
d. Sucrose
e. Charcoal
Answer:
Q. 3. Nitric acid is synthesised by oxidation of
a. Ammonia
b. Azo dye
c. Sodium nitrate
d. Hydrochloric Acid
Answer: Ammonia on catalytic oxidation gives nitric acid.
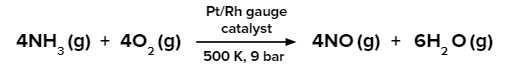
The nitric oxide is further oxidised to nitrogen dioxide which on hydrolysis produces nitric acid.
So, option A) is the correct answer.
Q. 4. In agriculture, the primary use of nitric acid is
a. Pesticide
b. Insecticide
c. Fertiliser
d. Herbicide
Answer: Nitric acid is used in fertiliser production to make various nitrogenous fertilisers such as calcium nitrate, ammonium nitrate, and others.
So, option C) is the correct answer.
Frequently Asked Questions - FAQ
1. Why does concentrated nitric acid not react with beryllium, aluminium, iron, or chromium?
Answer: Because of the creation of a protective oxide layer, the above four elements do not react with nitric acid. To make the reaction between these components and the nitric acid possible, this passive layer of oxide can be eliminated with salty water.
2. Does nitric acid stain the skin?
Answer: Skin turns yellow when exposed to nitric acid because skin proteins like keratin are transformed into yellow xanthoproteins.
3. When concentrated nitric acid combines with graphite and diamond, what happens?
Answer: When graphite reacts with concentrated nitric acid, mellitic acid is generated due to its oxidation, but diamond does not react with nitric acid.