-
Call Now
1800-102-2727
Preparation of Benzene– Characteristics of Benzene, Resonance in Benzene, Preparations, Properties, Uses, Practice Problem & FAQs
Benzene is like a synonym for organic chemistry! An important organic chemical that has a multitude of utilities. In fact production and usage of benzene can be considered as an important marker of industrial development of a nation.
The synthesis of benzene, a fundamental aromatic chemical, takes place in steel, petrochemical, and petroleum refineries. It is used to make a variety of downstream petrochemical products, including detergents, pesticides, resins, and nylon tyres.
Steel plants generate benzene from the coke oven gas in the corresponding aromatic recovery units. Various petrochemical units also produce benzene. So, let’s see what are the different methods to prepare benzene.
TABLE OF CONTENTS
- Benzene-Introduction
- Characteristics of Benzene
- Preparation of Benzene from Cyclic Polymerisation of Alkynes
- Preparation of Benzene from Aromatic Acids
- Preparation of Benzene from Sulphonic Acid
- Preparation of Benzene from Phenol
- Preparation of Benzene from Chlorobenzene
- Preparation of Benzene by Toluene Hydrodealkylation
- Preparation of Benzene from Cyclohexene and Cyclohexane
- Preparation of Benzene from Catalytic Reforming
- Preparation of Benzene from Aromatisation
- Preparation of Benzene from Diazonium Salt
- Preparation of Benzene from Grignard Reagent
- Physical Properties of Benzene
- Resonance in Benzene
- Uses of Benzene
- Practice Problems
- Frequently Asked Questions–FAQs
Benzene-Introduction
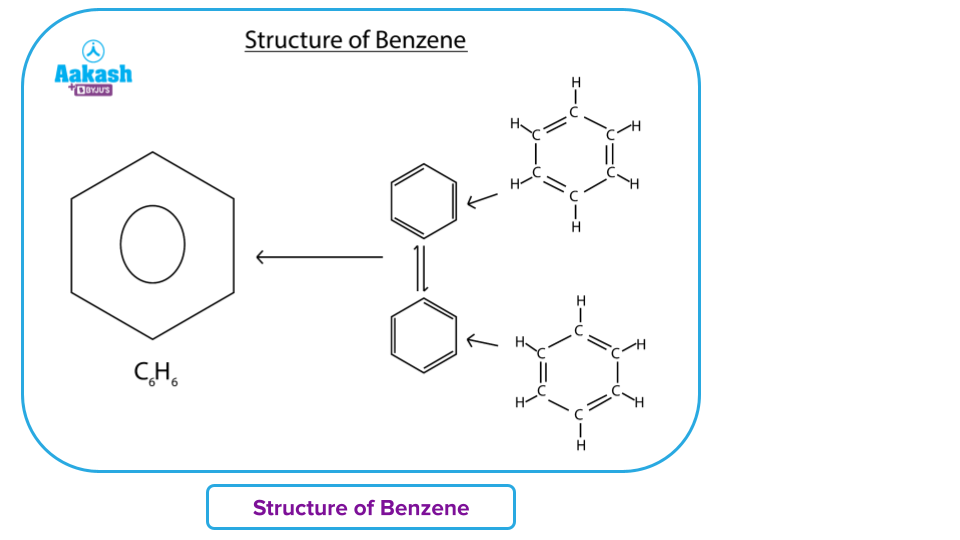
Cyclic hydrocarbons are the group to which benzene belongs. Benzene is the simplest aromatic hydrocarbon. Its chemical formula is C6H6. Each carbon atom in benzene forms a ring with six other carbon atoms. There is just one bond between each carbon atom and a hydrogen atom. The theory of valence bonds teaches us about the two stable resonance structures of the benzene ring, but the molecular orbital theory states that a benzene ring creates three - orbitals that stretch across all six carbon atoms.
The primary ingredient utilised in the production of several resins, synthetic fibres, plastics, pharmaceuticals, insecticides, rubber lubricants, detergents, and dyes is benzene. In its natural condition, benzene is produced by volcanoes and forest fires.
Benzene exhibits all the characteristics of aromaticity- i.e., it is conjugated, planar, cyclic and obeys Huckel’s rule’(that is, it contains 4n + 2 = - electrons) rule. Its structure is a hybrid of two equivalent resonating structures.
Characteristics of Benzene
English physicist Michael Faraday discovered benzene in 1825, and it wasn't until 1842 that it was made widely available once it was determined to contain benzene. Petroleum is currently used to extract large volumes of benzene.
A colourless liquid with the chemical formula C6H6, benzene has a distinctive smell. Benzene boils at 80.1oC and melts at 5.5oC. The essential chemical group known as aromatic compounds includes benzene and its derivatives. The manufacture of drugs, plastics, oils, synthetic rubber, and colours all use benzene as a precursor.
- It can catch fire.
- It has a poisonous character and is also flammable.
- It burns with a sooty flame.
- It smells like gasoline. It is a petrochemical product.
- The possibility of benzene causing cancer exists. It is classified as a carcinogen because of this reason.
- Because benzene and water cannot mix evenly, they are immiscible. It is soluble in a few organic solvents, though.
Preparation of Benzene from Cyclic polymerisation of Alkynes
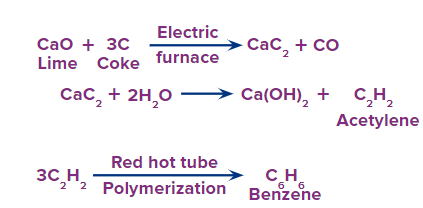
Ethyne can be converted to benzene by cyclic polymerization. The process of cyclic polymerization of acetylene when it passed through a red hot metallic tube at a very high temperature of 873 K, produces benzene.
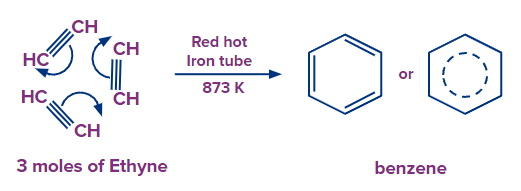
Ethyne can be obtained by hydrolysis of calcium carbide with water. CaC2+2H2O Ca(OH)2 + C2H2
Preparation of Benzene from Aromatic Acids
Aromatic acids are basically benzoic acid and its derivatives. Benzene can be obtained from the sodium salt of benzoic acid.
Generally in laboratories, benzene is simply made by boiling a soda lime and sodium benzoate solution. This process is known as decarboxylation. This technique involves heating sodium benzoate and soda-lime (sodium hydroxide combined with calcium oxide), which removes carbon dioxide and results in the production of benzene and sodium carbonate as a by-product.


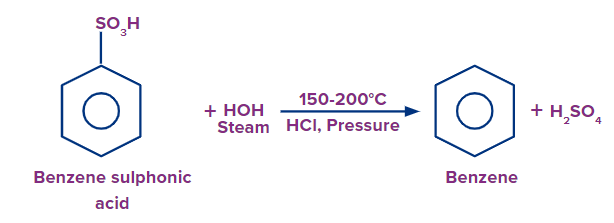
Preparation of Benzene from Sulphonic Acid
Through the hydrolysis of sulphonic acids, benzene can be produced. This procedure results in the creation of benzene by exposing benzene sulphonic acid to extremely heated steam. Upon boiling benzene sulphonic acid with dilute HCl acid under pressure at 150-200°C or hydrolyzing it with superheated steam yields benzene. This reaction is termed as desulphonation.
Preparation of Benzene from Phenol
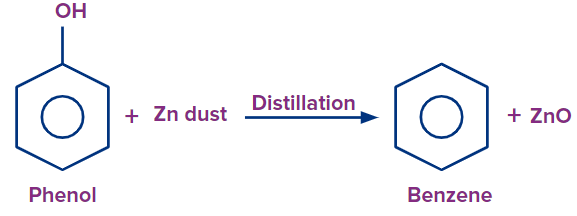
Benzene is produced when phenol is reduced with extremely hot zinc powder. Phenols can also be used to make benzene through reduction. In this procedure, heated zinc dust is passed over phenol vapours. They are reduced to benzene by zinc dust.

Phenol combines with zinc dust at a higher temperature to yield phenoxide ions and a proton, which then accept an electron from the Zn atom to form a H-radical, which is then used to create benzene. This causes the synthesis of ZnO and the self-conversion of the phenoxide ion into benzene.
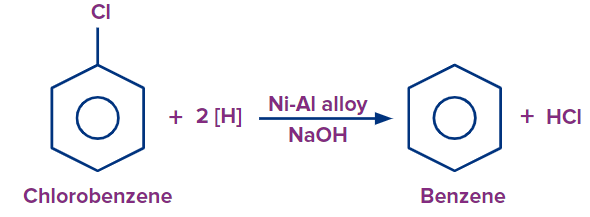
Preparation of Benzene from Chlorobenzene
Chlorobenzene on reduction with Ni-Al alloy and sodium hydroxide (NaOH) produces benzene.
Preparation of Benzene by Toluene Hydrodealkylation
Toluene hydrodealkylation converts toluene to benzene. In this hydrogen-intensive process, toluene and hydrogen are mixed, and the resulting mixture is then passed at 500–650 °C and 20–60 atm pressure over a catalyst made of chromium, molybdenum, or platinum oxide. Sometimes higher temperatures are used in place of a catalyst (at the similar reaction condition). The following conditions cause toluene to undergo dealkylation, turning it to benzene and methane..
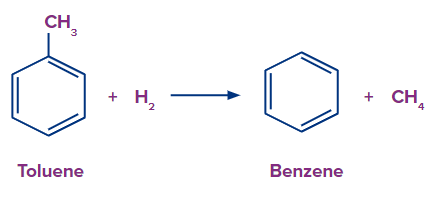
Typical yield is around 95 %.
Preparation of Benzene from Cyclohexene and Cyclohexane
Cyclohexene on reacting with ethene produces benzene and by-product ethane upon hydrogenation of ethene.
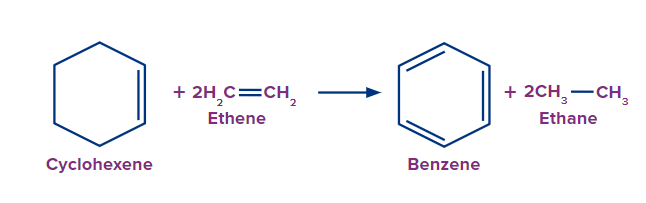 .
.
When cyclohexane is exposed to iron or quartz in a scorching-hot tube, it undergoes oxidation and transforms into benzene. This is known as oxidative dehydrogenation.

Preparation of Benzene from Catalytic Reforming
This is a very important industrial method of production of benzene in petrochemical industries.
- In catalytic reforming, hydrogen gas is combined with a variety of hydrocarbons having boiling temperatures between 60- 200 °C.
- At 500–525 °C and pressures ranging from 8–50 atm, the mixture is then subjected to a bifunctional catalyst like rhenium chloride or platinum chloride.
- In these circumstances, rings are formed and hydrogen is lost, converting aliphatic hydrocarbons to aromatic hydrocarbons.
- Following extraction using any one of a variety of solvents, such as diethylene glycol or sulfolane. By using distillation, benzene is then separated from the other aromatic compounds after the aromatic reaction products have been isolated from the reaction mixture (or reformate).
- The reformate aromatics extraction phase aims to create aromatics with the fewest non-aromatic components possible. The process of recovering the aromatics, often known as BTX (benzene, toluene, and xylene) extraction.
Let’s see an example:
The process of the formation of aromatic hydrocarbons from alkenes when heated at high temperatures and pressure conditions is catalytic reforming. Through this technique cyclohexene in reaction with ethene can produce benzene.

Preparation of Benzene from Aromatisation
n-Hexane on aromatisation produces benzene. The process occurs in the presence of chromic oxide-alumina catalyst at 600oC and 15 atm.

Preparation of Benzene from Diazonium Salt
H3PO2 or CH3CH2OH reacts with benzene diazonium salts to produce benzene. The reagent hypophosphorous acid is a reducing agent and thus it reduces -N2+Cl-salt to -H. So we obtain benzene.

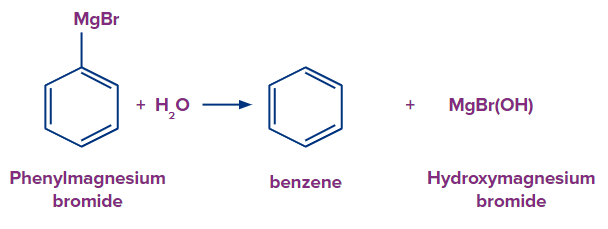
Preparation of Benzene from Grignard Reagent
Any phenyl magnesium halide (Ph-Mg-X) will hydrolyze in an acidic media to produce benzene as the end result. Benzene forms when diluted sulfuric acid and phenyl magnesium chloride are combined.
Physical Properties of Benzene
- Its chemical formula is C6H6.
- It dissolves in organic solvents but is insoluble in water. Additionally, benzene and water do not combine uniformly.
- It is an odourless & colourless, liquid aromatic cyclic molecule with a colour similar to gasoline.
- It is extremely stable due to the fact that it enumerates resonance and can take on different forms, most of which rely on the location of the double bond.
- The resonance energy of benzene is 36 kcal mol-1.
- In the presence of air, it easily catches fire. We can therefore describe it as being highly flammable, and when it burns, a comforting flame is created.
- The boiling point of benzene is 80.5C.
- The density of benzene is around 0.87 g mL-1.
- The melting oint of benzene is 5.5C.
- The molar mass of benzene is 78 g mol-1.
Resonance in Benzene
Three carbon-carbon double bonds (C=C) and a six carbon-carbon single bonded ring (C-C), symbolised by a hexagon, are present in the structure.
As opposed to a C-C bond, a C=C bond is shorter. C-C bonds have lengths that are longer than double bonds (135 pm) but less than single bonds (147 pm). But all six of the carbon-carbon bonds in benzene have the same length, which is in the middle of a single bond and a double bond's ranges. It is a regular hexagon and is planar.
The single bonds, also known as -bonds, are produced by the overlap of hybridised atomic sp2 orbitals in the plane between the carbon nuclei. A -bond and a -bond make up the C=C double bonds. The atomic p- orbitals above and below the plane of the ring overlap laterally to generate the -bonds.

So within the ring the circular -electron density is evenly distributed through a - bond above and below the ring. This is explained as resonance.
Three double bonds and three single bonds, represented as 1,3,5-cyclohexatriene or 2,4,6-cyclohexatriene, make up the conventional representation of the benzene structure. The actual structure of benzene resembles a combination of the two.
As a result, each side actually creates a relationship that is a compromise between single and double security, which continues to sway inside the ring. Every carbon atom that is present within this ring has undergone sp2 hybridization. Due to the presence of two sp2 hybridised orbitals, one of them connects to the nearby carbon molecule sp2 hybridised orbital to form a C-C bond.
A C-H bond is created when the next sp2 hybridised orbital attaches to the hydrogen s-orbital. Consequently, six C-C and six C-H sigma bonds were formed.
Uses of Benzene
- Aniline and phenol, both of which are utilised in colours, are produced using benzene.
- Nylon fibres are also made with the help of benzene.
- Additionally, benzene is used to make nitrobenzene, cumene, and ethylbenzene.
- Benzene is rarely used in the household due to its toxic and carcinogenic qualities.
- Additionally, it is used to produce tobacco, cleaning supplies, glue, and other adhesives.
- It is a chemical reagent used in the production of pigments, pesticides, soaps, cleansers, engineered filaments, and plastics.
- Benzene can also be acquired naturally through volcanic eruptions and forest fires.
- In the case that it overflows from full tanks, it disappears fast from water and soil. However, it is hazardous and may result in the contamination of water wells and water supplies.
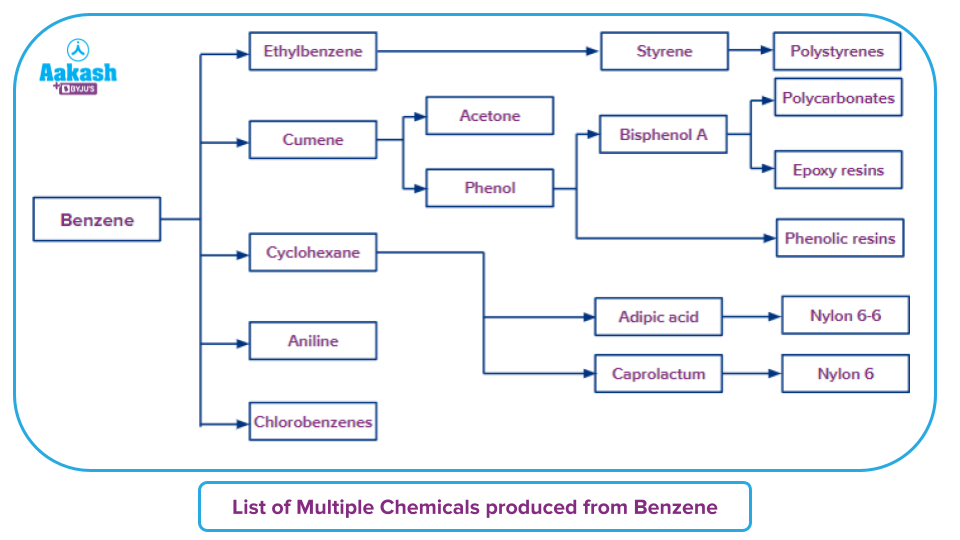
Here is a list of multiple kinds of elements prepared from benzene:
Recommended Video
https://www.youtube.com/watch?v=nIwCq2oiOEw
Practice Problems
Q.1. Can we produce benzene from lime? If yes, elaborate.
Answer: If we can obtain ethyne from lime, we shall be able to convert ethyne to benzene by cyclic polymerisation.
Here is the elaborated procedure:
Q.2. Benzene can be obtained from phenol by the action of zinc dust. What is the role of Zn here?
- Oxidising agent
- Reducing agent
- Catalyst
- Initiator
Answer: (B)
Solution: Zinc dust acts as a reducing agent. Phenol combines with zinc dust at a higher temperature to yield phenoxide ions and a proton, which then accept an electron from the Zn atom to form a H-radical, which is then used to create benzene. This causes the synthesis of ZnO and the self-conversion of the phenoxide ion into benzene. So option B is the correct answer.
Q.3. What is the state of benzene at STP?
- Liquid
- Solid
- Gas
- Plasma
Answer: (A)
Solution: Benzene is obtained as a solid at STP. Benzene is a liquid at room temperature since it has a melting point of 5.5°C and a boiling point of 80°C and below 5.5 °C it is in solid state as the temperature at STP is 0 °C. Hence the correct answer is option B.
Q.4. Convert benzaldehyde to benzene?
Answer: First benzoic acid is created when benzaldehyde is subjected to oxidation in the presence of alkaline potassium permanganate. Benzene is produced when soda lime, a combination of NaOH and CaO, and benzoic acid are heated together.

Frequently Asked Questions–FAQs
Q.1. How is steam cracking beneficial in industrial production of benzene?
Answer: In petrochemical industries and oil refineries, aliphatic hydrocarbons are converted into ethylene and other alkenes by the process of steam cracking. Steam cracking can result in the production of pyrolysis gasoline, a liquid by-product rich in the benzene found in the feedstock used to make the olefins.
In order to recover BTX aromatics (benzene, toluene and xylenes), pyrolysis gasoline can either be extracted or combined with other hydrocarbons as a gasoline additive.
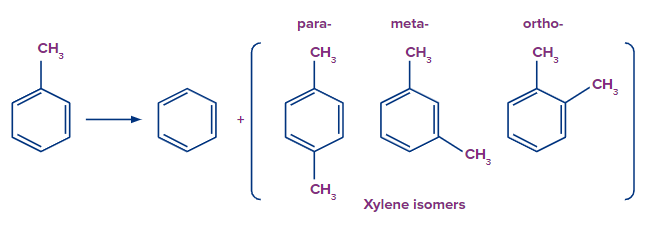
Q.2. What is TDP?
Answer: Toluene is converted to benzene and xylene by a process known as toluene disproportionation (TDP). It is a trans-alkylation process. Toluene disproportionation is a crucial industrial process for converting less valuable toluene into more commercially valuable p-xylene or o-xylene and benzene.
Q.3. Is benzene carcinogenic?
Answer: Benzene is a known cause of bone marrow failure and is categorised as a carcinogen, which raises the risk of cancer and other disorders. A large body of epidemiological, clinical, and laboratory evidence connects benzene to cardiovascular illness, aplastic anaemia, acute leukaemia, abnormalities of the bone marrow, and acute leukaemia.
Q.4. What is BTX extraction with respect to obtaining benzene industrially?
Answer: The initials BTX are used in the petrochemical and petroleum refining sectors to designate mixtures of the aromatic hydrocarbons benzene, toluene, and the three isomers of xylene.
There are numerous ways to produce benzene, toluene, and xylenes. However, the majority of BTX is produced by recovering aromatics from naphtha that has been catalytically reformed in a petroleum refinery. About 44–50% of the total benzene produced in the US was from catalytic reformates.
BTX is required for the extraction of the production of in-demand chemicals like benzene and nylon, which are used to make anything from glue to pharmaceuticals. Naphtha, a combustible mixture made from natural gas, oil, and coal, is one of the main ways to make BTX


