-
Call Now
1800-102-2727
Preparation of Phenols: Structure, Classification, Preparations of Phenol, Practice Problems & Frequently Asked Questions
Consider a hospital full of patients getting treatment for various infectious and contagious diseases. Are we not worried, such infectious diseases should not spread to others?
What methods are employed to keep the hospital clean?
Moreover, are we not interested in keeping our house free from such disease-causing germs and insects?
The liquids used to keep the hospital and household, clean should be a disinfectant and an antiseptic
Phenol is one such simple and cheap disinfectant cum antiseptic. It is effective against a wide variety of microorganisms, including some fungi and viruses, but only gradually against spores. Phenol has traditionally been used to clean the skin and reduce itching. Pure and dilute phenol is employed in various medical procedures, as well as in a variety of treatments and laboratory applications.
In molecular biology, phenol-chloroform mixture is used as an extraction medium to extract nucleic acids from biological samples. Variation of pH of the solution, either DNA or RNA can be isolated.
Let us see the characteristics and preparation of phenols
Table of contents:
- Structure of phenol
- Classification of phenol
- Preparations of phenol
- Practice problems
- Frequently asked questions(FAQs)
Structure of phenol:
Phenol is an organic molecule that has an -OH group attached to a benzene ring.The chemical formula of the first homologous phenol is C6H5OH .

All the six carbon atoms of the benzene ring of phenol are sp2 hybridised. As a result, phenyl ring has a hexagonal planar structure with 120o bond angles between carbons. The three pairs of pi-electrons are in conjugation and hence delocalized over all the carbon atoms..
The O-H bond is polar due to the higher electronegativity of O over H. .
The nonbonded electrons in oxygen are in conjugation with the benzene ring. A pair of oxygen nonbonded electrons can undergo resonance with the benzene ring. This creates a partial positive charge on oxygen. Positively charged oxygen can pull stronger the O-H bond electrons facilitating the loss of H as proton.Because of the resonance in the benzene ring,the phenolic O-H is more acidic than alcoholic O-H bond.
But phenol is still a weak acid for the fact the resonance stabilises equally the ionised (phenolate ion) and unionised phenol..
The dispersion of the negative charge of the phenolate ion over the molecule can be depicted using resonance structures or as a resonance hybrid, as seen in Figure
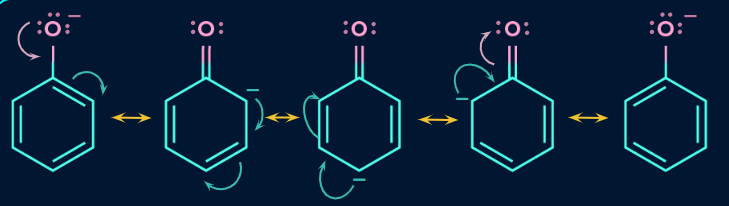
Classification of phenol:
Phenol is an organic molecule that has at least one -OH group linked directly to the benzene ring. The number of hydroxyl groups linked to the benzene ring determines whether a phenol is monovalent, divalent, or trivalent.
Monovalent phenol: The simplest member of this class is hydroxybenzene, sometimes known as phenol, while others are known as substituted phenols. Ortho cresol, meta cresol, and para cresol are the three isomeric hydroxy toluenes.
Dihydric phenol: Dihydroxybenzenes (benzene diols) are organic chemical compounds formed by substituting two hydroxyl groups onto a benzene ring.
Dihydroxybenzene: The common names for three isomers of dihydroxybenzene, namely catechol, resorcinol, and quinol, are well known by their common names.
Trihydric phenol: Trihydroxybenzene (benzene triols) are organic chemical compounds that have three hydroxyl groups substituted onto a benzene ring.
Trihydroxybenzene: The common names for three isomers of trihydroxybenzene are pyrogallol, hydroxyquinol, and phloroglucinol.
Preparations of phenol:
The following methods can be used to synthesise phenol.
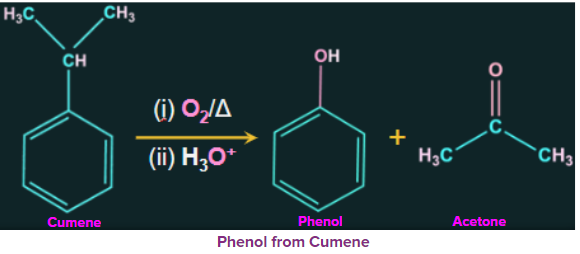
1. Preparation of phenol from cummene:
By Friedel-Crafts alkylation of benzene with propylene, cumene is an organic compound produced. Cumene (isopropylbenzene) is oxidised in the presence of air to produce cumene hydroperoxide. Phenol is produced by further treating cumene hydroperoxide with dilute acid. One of the by-products of this process is the production of acetone in large amounts. Therefore, it is necessary to purify the phenol that results from these processes.
2. Production of phenol from benzene sulphonic acid:
Benzene reacts with oleum, and benzenesulfonic acid is produced. At high temperatures, this benzenesulfonic acid can be treated with molten sodium hydroxide to promote the synthesis of sodium phenoxide. Finally, with acidification, sodium phenoxide yields phenol.

3. From aromatic primary amines:
The diazonium salt is produced by treating aromatic primary amines with freshly prepared nitrous acid (NaNO2+HCl) at 273-278 K. These diazonium salts are quite reactive by nature. These diazonium salts finally hydrolyze to phenol when heated with water. Treatment with dilute acids can also yield phenol from diazonium salts.

4. Dow’s process:
Dow's procedure is a phenol preparation method. To produce sodium phenoxide ion, the reactant chlorobenzene is heated with aqueous sodium hydroxide at temperatures of 623 K and 300 atm. The sodium phenoxide ion is then treated with dilute HCl, yielding the end product, phenol.

Practice problems:
Q.1. Which one of the following phenols has the highest PKa value?
(A) 2,4,6 Trinitro phenol
(B) P -Cresol
(C) m-Nitrophenol
(D) None of the above
Answer : (B)
Solution: The presence of the electron-withdrawing benzene ring in phenol makes it acidic because it stabilises the phenoxide ion generated after proton removal. Electron-donating groups, on the other hand, reduce the acidic character of phenol. More acidic strength,less PKa value. More PKa value, less acidic strength. Cresol (C7H8O), any of the three methyl phenols with the same molecular formula but different structures: ortho- (o-) cresol, meta- (m-) cresol, and para- (p-) cresol, has a higherPKa value than the others due to the presence of the electron-donating group methyl, so P-Cresol has the highest PKa value.
Q.2. Which alcohol is less acidic?
(A) C2H5OH
(B) (C2H5)2CHOH
(C) (C2H5)3COH
(D) none of the above
Answer: (C)
Solution: Primary alkoxide ions are the most stable and tertiary alkoxide ions are the least stable when considering both electronic and steric factors. Because of this, primary alcohols have the highest acidity character, whereas tertiary alcohols have the lowest acidity character.
Thus, the descending sequence of alcohol acidity is as follows:
Primary alcohol (1o) >Secondary alcohol (2o) >Tertiary alcohol (3o)
Q.3. The correct order of acidic strength in water of the given substituted phenols
(A) p-nitrophenol > p-fluorophenol >p-chlorophenol
(B) p-nitrophenol> p-chlorophenol > p-fluorophenol
(C) p-chlorophenol> p-fluorophenol> p-nitrophenol
(D) All of the above
Answer: (B)
Solution: The sequence of electron withdrawing tendency from the benzene ring is as follows:
As the tendency to remove electrons grows, so does acidic strength.
The stability of the anion after losing the hydrogen atom determines the acid strength. Both inductive and mesomeric actions influence the compound's acidic strength. All three groups are electron withdrawing groups.
The inductive effect is stronger in halogens, but the mesomeric effect is stronger in other cases. Nitro group, demonstrating that -M dominates all other effects. When this is introduced to any group, the electron cloud is considerably reduced.
The inductive effect is present in both fluoro and chloro. When the resonant structures of p-chlorophenol and p-fluorophenol conjugate bases are studied, we can see that the p-chloro phenoxide ion has an extra resonance structure. This happens because chlorine atoms have an extra unoccupied d-orbital for electron delocalization that fluorine atoms do not. As a result, the p- chlorophenol conjugate base is more stable and consequently acidic. P-chlorophenol has a higher acidity than p-fluorophenol.
Q.4. 2-hydroxy benzoic acid (salicylic acid)contains
(A) Intramolecular hydrogen bonding
(B) Intermolecular hydrogen bonding
(C) Both A and B
(D) None of the above
Answer: (A)
Solution: All of the oxygen atoms in salicylic acid will have a partial negative charge because oxygen has a lower electronegativity than the atoms around it. The most electronegative and accessible of all the atoms is the oxygen, which has a double bond with the carbonyl carbon in the acid group. The hydrogen atom in the hydroxyl group will have a partial positive charge since oxygen is present. There will be a hydrogen bond between these two atoms.

As we can see, 2-hydroxybenzoic acid forms intramolecular hydrogen bonds, but 4-hydroxybenzoic acid forms intermolecular hydrogen bonds and hydrogen bonds with other molecules.
Frequently asked questions(FAQs):
Q1. What makes phenols less acidic than carboxylic acids?
Answer: Carboxylic acid, phenols and alcohols all have hydroxyl O- H group. But the ease of release of the hydrogen from O varies among them. Molecules releasing hydrogen ion are called acids. Acid are graded according to their ability to release these hydrogen ions. The ease of release of hydrogen depends on the nature of the groups linked to hydroxyl oxygen. Electron donating groups like alkyl reduce the release of hydrogen ions and hence have low acidic character as shown by alcohols. On the other hand electron withdrawing groups shall increase the acidity.
The phenyl group of phenol has an electron withdrawing nature due to the formation of resonance structures that stabilises the phenolate ion. So phenols are more stronger acid than alcohols. The resonanc structure also stabilises the unionised phenol, reducing the acidity of phenol.
In carboxylic acid, the carboxylate ion is also stabilised by resonance. But the presence of two electronegative oxygen atoms and identicality of the resonance structure makes very strong acidic nature of carboxylic acids.
Q2. Why doesn't phenol produce quick effervescence when combined with sodium bicarbonate?
Answer: Phenol is naturally acidic. However, it is a weak acid. The presence of -OH on phenol and electron conjugation in benzene rings both pull electrons away from the -OH group. A weak base is sodium bicarbonate. It readily takes electrons from stronger acids such as carbonic acid, but it lacks the strength to remove the proton from phenol. As a result of phenol's mild acidic nature, NaHCO3 does not react with it and doesn’t produce quick effervescence.
Q3. In phenol, the carbon-oxygen bond is somewhat stronger than in methanol. Why?
Answer: This derives from the fact that in phenol, conjugation of an oxygen unshared electron pair with an aromatic ring gives the partial double bond nature carbon-oxygen.
In methanol, oxygen is linked to a carbon atom which is SP3 hybridised, as opposed to a phenol SP2 hybridised carbon atom.The bond formed by SP2 hybridised carbon and oxygen is stronger than the bond formed by SP3 hybridised carbon and oxygen.
Q4. What exactly are phenols? What distinguishes them from alcohol?
Answer: Phenols are compounds that have a -OH directly linked to a benzene ring. In phenol, the hydroxyl group (-OH) is connected to a SP2 hybridised carbon atom, whereas hydroxyl group (-OH) are attached to an SP3 hybridised carbon atom in aliphatic alcohols. A phenolic -OH is distinguished by the emergence of violet/purple coloration in the presence of a neutral FeCl3 solution. Phenol is an aromatic chemical molecule with the molecular formula C6H5OH, also known as carbolic acid.


