-
Call Now
1800-102-2727
Carbohydrates: Overview, Monosaccharides, Practice Problems and FAQs
Carbohydrates: General structure, Classification, Practice Problems and FAQs
Carbohydrates are very popular these days for not being part of the diet of those on the journey of weight loss. You must have heard about the “no carb diet”. Well carbohydrates are an essential part of our diet, as we know that for a healthy lifestyle a balanced diet is a must which very much includes carbohydrates. People do not have a clear idea about carbohydrates, therefore it is vital to remember that eating carbohydrates from nutritious meals is more crucial than adhering to a rigid diet that restricts or counts the amount of carbohydrates taken.The type of carbohydrate you choose to consume is crucial since some sources are healthier than others. The type of carbohydrates in the diet is more significant than their quantity, whether high or low. However, what are these carbohydrates? Let’s find out!
Table of Contents
- What are carbohydrates
- General structure
- Classification of carbohydrates
- Monosaccharides
- Disaccharides
- Oligosaccharides
- Polysaccharides
- Reducing and non reducing sugars
- Benedict’s test for reducing sugars
- Practice Problems
- FAQs
What are carbohydrates?
Carbohydrates include all the sugar and starch you have heard of in your food. When you burn sugar, you get carbon and water. That is how the scientists thought of the name carbohydrates or hydrates of carbon. These are the compounds having carbon, hydrogen and oxygen in the ratio 1:2:1.

Fig: Sources of carbohydrates
General structure
Carbohydrates have at least 3 carbon atoms, multiple hydroxyl (-OH) groups and either an aldehyde (-CHO) group or a ketone (C=O) group. If an aldehyde group is present then it is called an aldose and if a ketone group is present then it is called a ketose. For example, glucose is an aldose sugar with six carbons and fructose is a ketose sugar with six carbons. However, both of them have the same molecular formula.

Fig: General structure of carbohydrates
Carbohydrates as you know are defined as hydrates of carbon. Thus, the general formula for carbohydrates is Cn(H2O)n. However there are a few rebels, as in sugars that do not follow the general formula for carbohydrates. For example Rhamnose (C6H12O5) and Deoxyribose (C5H10O4).

Fig: Carbohydrates that do not follow the general formula
Classification of carbohydrates
Carbohydrates are classified based on the number of monomeric units into the following types:
- Simple sugar
- Monosaccharides
- Disaccharides
- Complex sugar
- Oligosaccharides
- Polysaccharides

Fig: Classification of carbohydrates
Monosaccharides
Mono = Single; Saccharide = Sugar. These are the simplest carbohydrates which are generally sweet in taste. They are the building blocks of larger carbohydrates and cannot be further hydrolysed to smaller sugar units.
Monosaccharides are further classified based on the number of carbon atoms.
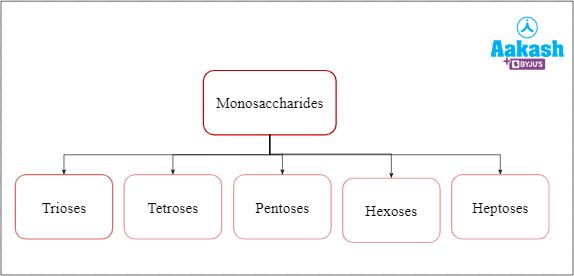
Fig: Classification of monosaccharides
Monosaccharides can have a maximum of 10 carbon atoms but the ones with 8 (octose), 9 (nonose) or 10 (decose) carbon atoms are not commonly found.
Triose
Trioses are monosaccharides with 3 carbon atoms. Eg: glyceraldehyde (C3H6O3)
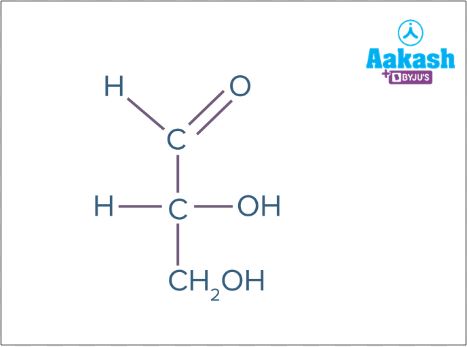
Fig: Glyceraldehyde
Tetroses
Tetroses are monosaccharides having 4 carbon atoms. Eg: D-Erythrose (C4H8O4)

Fig: Erythrose
Pentoses
Pentoses have 5 carbon atoms. Eg: D-Ribose (C5H10O5), D-Deoxyribose (C5H10O5)
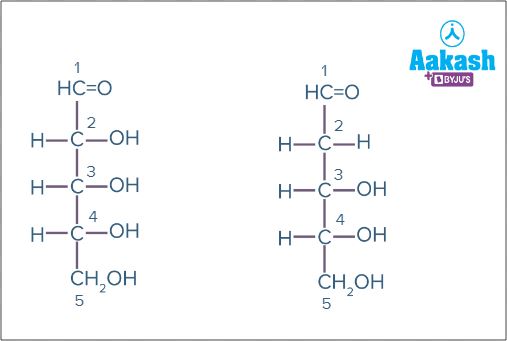
Fig: D-Deoxyribose Fig: D-Ribose
(no OH at carbon 2) (OH on carbon 2)
Hexoses
Hexoses have 6 carbon atoms. Eg: glucose, fructose, galactose
- Glucose is an aldose sugar with an aldehyde group. It is the most important source of energy for all the organisms. It is the most abundant monosaccharide. It is called by many names- blood sugar, grape sugar, corn sugar, dextrose (dextrorotatory in nature).
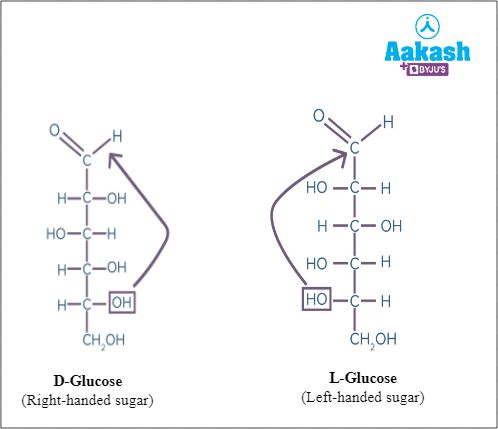
Fig: Glucose
- Fructose is a sugar that contains a ketone group, so it is a ketose sugar. It is the sweetest naturally occuring sugar. It is also called ‘fruit sugar’ because it is the major sugar in all the fruits. ( Exception : Grapes, where the major sugar present is glucose).

Fig: Fructose
Enantiomers
Monosaccharides have two optically active forms called enantiomers of each other. If the OH on the bottom chiral centre points to the right, it is referred to as D- . If the OH on the bottom chiral centre points to the left, it is referred to as L- .These optically active forms are Non-superimposable mirror images.
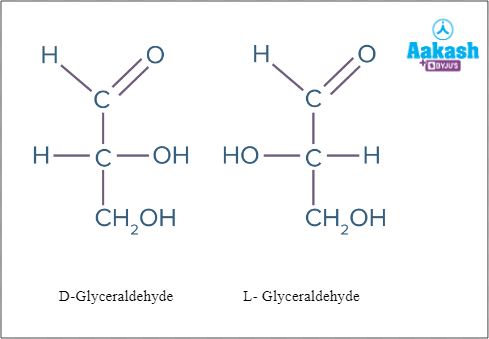
Fig: Enantiomers of glyceraldehyde
If light is allowed to pass through some filter like a prism (polariser), then after passing through it, the light waves vibrate only in one plane. This light is called plane polarised light. Dextrorotatory (d) turns the plane polarised light to the right and laevorotatory (l) turns the plane polarised light to the left.

Fig: Plane polarised light
Disaccharides
Di = Two; Saccharide = Sugar. Disaccharides consist of two monosaccharide units which are joined with a glycosidic bond. Eg: sucrose, maltose, lactose, etc.
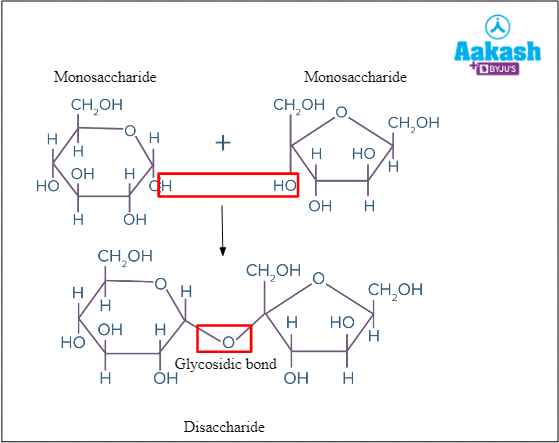
Fig: Formation of Disaccharide
- Sucrose = glucose + fructose. It is also called ‘table sugar’ or ‘cane sugar’ as it is commercially obtained from Saccharum officinarum (Sugarcane). Sucrose occurs as storage carbohydrate in sugarcane and sugar beet.
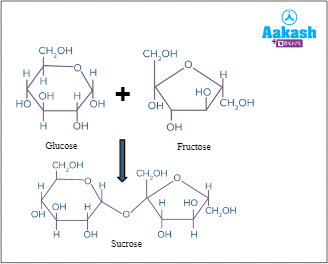
Fig: Sucrose
- Maltose= glucose + glucose. It is also called ‘malt sugar’ as it is commonly obtained from malted cereal grains. Malting is a process which includes germinating and then drying of cereal grains. Other sources include Sweet potatoes, beer ( made from malted barley), corn syrup, etc.

Fig: Maltose
- Lactose= glucose + galactose. It is also called milk sugar as it is found in milk and milk derived products.

Fig: Lactose
Oligosaccharides
Oligo’ = Few; ‘Saccharide’ = Sugar. In oligosaccharides 3-9 monosaccharide units are joined by glycosidic linkage. Examples include:
- Trisaccharide: 3 monosaccharide units
- Raffinose = Glucose + Galactose + Fructose
- Tetrasaccharide: 4 monosaccharide units
- Stachyose = Glucose + Galactose + Galactose + Fructose
Raffinose can be found in beans, cabbage, brussels sprouts, broccoli, asparagus, other vegetables, and whole grains.Stachyose occurs naturally in numerous vegetables and other plants.
Polysaccharides
Polysaccharides are polymers of repeating units of monosaccharides and are also called ‘glycans’. They are the macromolecules and have high molecular weight.
They have a non-reducing end where there are no free reactive groups and a reducing end where a free reactive group is present.
Polysaccharides are classified based on whether the monomeric units are of same type or different
- Homopolysaccharides
- Heteropolysaccharides
Homopolysaccharides
Homopolysaccharides have the same type of monomeric units. They are further classified into structural polysaccharides which form the structural part of living organisms. Eg:- cellulose and chitin and storage polysaccharides are used for storage. Eg: starch, glycogen, inulin
- Cellulose - It is the most abundant biomolecule on the earth and forms cell wall components in plants and protists. It is the polymer of glucose units containing repeating glucose units in a straight chain and is unbranched.

Fig: Cellulose
- Chitin - It is the second most abundant organic substance.It is present as a structural component in the exoskeleton of insects and fungal cell walls. It consists of repeating units of a nitrogen containing glucose derivative called N- acetyl glucosamine(NAG).

Fig: Structure of chitin
- Starch - It is a polymer of glucose and serves as the food reserve in plants. It consists of amylose - 20% - 30% and amylopectin- 70% - 80%. Amylose is a straight chain polymer in which glucose units are joined by α-1,4 glycosidic bonds. Amylopectin is a branched chain polymer where the regular straight chains have glucose units joined by α-1,4 glycosidic bonds and the branch points have α-1,6 glycosidic bonds.

Fig: Amylose and amylopectin
- Glycogen - It is a highly branched polymer of Glucose. It serves as the food reserve in animals and is stored in the liver and muscles. Glycogen also has a branched structure in which the branch points have α-1,4 glycosidic bonds and the regular straight chain has α-1,6 glycosidic bonds.
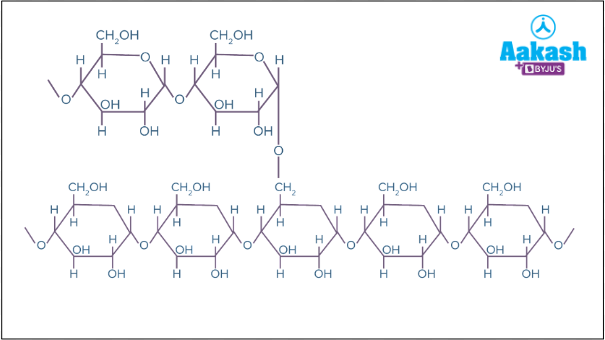
Fig: Structure of glycogen
- Inulin - It is the polymer of fructose. It is stored in the roots of Dahlia, Dandelion and Artichoke. It cannot be metabolised by the human body and filtered as such by the kidney. It is also used to test the rate of filtration occuring in the kidney and related functions.
Heteropolysaccharide
Heteropolysaccharide consist of different types of monomeric units. Eg: agar and peptidoglycan.
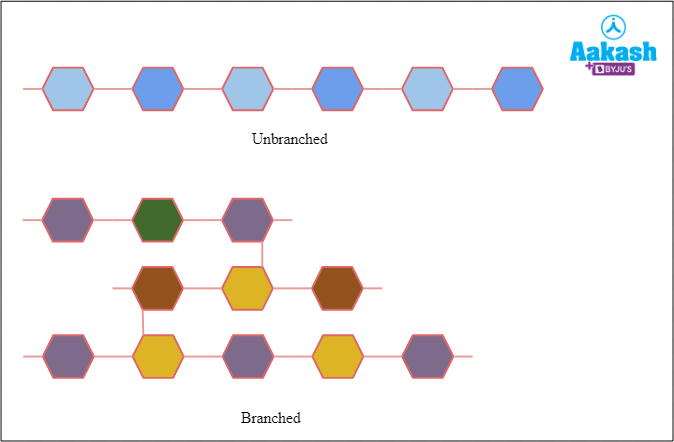
Fig: Unbranched and Branched heteropolysaccharides
- Peptidoglycan-it is the component of the bacterial cell wall. It is made of two different kinds of repeating units N- Acetyl glucosamine (NAG) and N-Acetyl muramic acid (NAM).

Fig: Structure of peptidoglycan
- Agar - It is obtained from the red algae - Gelidium and Gracilaria. It is made of two different units- Galactose and 3,6-anhydro-L-galactopyranose.
Reducing and non reducing sugars
Reducing sugars are those which have a free aldehyde/ketone group present. Eg: All monosaccharides, disaccharides like maltose and lactose are reducing sugars.
Non-reducing sugars are those which do not have a free aldehyde/ketone group. All monosaccharides are reducing sugars and all polysaccharides and disaccharide like sucrose are non-reducing.
Benedict’s test for reducing sugars
Benedict’s test is a test for reducing sugars. Reducing sugars reduces the cupric ions in Benedict’s solution to cuprous, while non-reducing sugars cannot.
When reducing sugar is added to benedict’s reagent and heated it results in the formation of a brick red precipitate.
This principle is applied in testing the presence and amount of glucose in blood and urine.

Fig: Benedict’s test for reducing sugar
Practice Problems
Q1. Glucose is produced from inorganic sources by:
- Some bacteria, algae and green plants
- Fungi, algae and green plant cells
- All bacteria, fungi and algae
- Viruses, fungi and bacteria
Solution: All carbohydrates are derived from glucose via different metabolic pathways and photosynthetic organisms are the only living beings which synthesise glucose via the process of photosynthesis. Thus photosynthetic bacteria, and chlorophyll containing organisms such as algae and green plants are the only organisms which can produce glucose from inorganic substrates such as carbon dioxide and water, in the presence of water, via the process of photosynthesis. Thus, the correct option is a.
Q2.Which one of the following is a non - reducing carbohydrate?
- Maltose
- Sucrose
- Lactose
- Ribose 5- phosphate
Solution: Carbohydrates that have a free aldehyde or keto group are called reducing sugars. Whereas if there is no free aldehyde or keto group then the carbohydrate is called a non- reducing sugar. Sucrose is an example of a non-reducing sugar. Maltose, lactose and ribose 5- phosphate are reducing sugars. Thus, the correct option is b.
Q3. Chitin is a
- Phosphorus-containing polysaccharide
- Sulphur containing polysaccharide
- Simple polysaccharide
- Nitrogen-containing polysaccharide
Solution: Chitin is a nitrogen-containing polysaccharide that constitutes the cell wall of fungi and exoskeleton of arthropods. Chemically chitin is a polymer of N- acetylglucosamine. Chitin contains neither phosphorus nor sulphur. Chitin is not a simple polysaccharide. Thus, the correct option is d.
Q4. Lactose is composed of
- Glucose+Glucose
- Glucose+Fructose
- Glucose+Galactose
- Fructose+Galactose
Solution: Lactose is a disaccharide and is made up of glucose and galactose. It is found in milk and milk products. Glucose and galactose are joined together by a beta glycosidic bond to form lactose. Thus, the correct option is c.
FAQs
Q1. What is the composition of Benedict's reagent?
Answer: Benedict's reagent is an alkaline solution of blue coloured copper(II) sulphate pentahydrate, sodium carbonate, and sodium citrate which is used as a chemical reagent. It is frequently used to find reducing sugars. When a Benedict’s reagent along with a reducing sugar is heated it results in the formation of red coloured insoluble precipitate of copper (I) oxide.
Q2. How can fructose and glucose be distinguished?
Answer: Fructose and glucose can be distinguished by Seliwanoff’s reagent. It is a test which helps in distinguishing between an aldose and a ketose sugar. Fructose is a ketose sugar, whereas glucose is a hexose sugar.
Q3. In maltose, the glycosidic bond forms between which two carbon atoms of the adjacent monosaccharide subunits?
Answer: Maltose is a disaccharide which is formed by two units of glucose molecules linked by a glycosidic bond. The bond is formed between the C1 of one glucose with the C4 of another glucose molecule.
Q4. Which sugar is found in cereales and candies
Answer: Maltose is a sugar found in some cereals and candies. It is a product glucose+glucose and may be purified from barley and other grains. In the manufacture of beer, maltose is liberated by the action of malt (germinating barley) on starch therefore, it is often referred to as malt sugar.
Q5. Why are carbohydrates referred to as “Staff of life”?
Answer: Carbohydrates are referred to as the staff of life as they are the most basic components of food that serve as the primary source of energy to all living organisms.
YOUTUBE LINK: https://youtu.be/ZcDIetuGKEk



