-
Call Now
1800-102-2727
Ozone: Formation and Depletion in the Stratosphere, Practice Problems and FAQs
Have you heard about ozone? Yes, in lower classes we learned that it protects us from harmful radiations. The ozone shield is located in the Earth’s stratosphere. You know, it is able to absorb about 98 percent of the harmful ultraviolet radiation!!

Fig: Ozone layer
Without ozone, the Sun’s intense UV radiation would sterilise the Earth’s surface and we won’t be able to survive on this Earth. The most energetic, UV-C radiation, and most of the UV-B radiation are screened by the ozone layer. But increased levels of human-produced gases such as CFCs (chlorofluorocarbons) have led to increased rates of ozone destruction and its depletion. This upsetting the natural balance of ozone and leading to reduced stratospheric ozone levels.
The reduced ozone levels in the atmosphere has increased the amount of harmful radiations like ultraviolet rays reaching the Earth’s surface. You might have heard about the ozone hole. When scientists talk about the ozone hole, they are talking about the destruction of stratospheric, ‘good ozone.’ Let us understand more about the ozone layer and effects of its depletion in detail in this article.
Table of contents
- Atmosphere
- Layers of atmosphere
- Ozone layer
- Effect of UV rays
- Formation of ozone Layer
- Depletion of ozone Layer
- Practice Problems
- FAQs
Atmosphere
The blanket of air present around the Earth is called the atmosphere. It is required to sustain all the types of life forms on the Earth. The atmosphere can be divided into different layers. It consists of the exosphere, thermosphere, mesosphere, stratosphere, and troposphere.
Layers of atmosphere
Based on its temperature, the atmosphere is divided into different layers. These layers are as follows :
Troposphere
This is the lowest part of the atmosphere where organisms live. It contains most of the clouds, rain and snow.
Stratosphere
This layer extends upwards from the troposphere to about 50 km. It possesses most of the ozone in the atmosphere. The increase in temperature with height occurs in this region because of absorption of ultraviolet (UV) radiation from the sun by ozone.
Mesosphere
It is the region above the stratosphere. Here the temperature decreases with height, reaching a minimum of about -90°C.
Ionosphere
It is the region of the atmosphere above about 80 km. It is part of the thermosphere and is called the ionosphere as the energetic solar radiation knocks electrons off molecules and atoms, turning them into ‘ions’ with a positive charge.
Thermosphere
It lies above the mesosphere and is a region in which temperatures again increase with height. This temperature increase is caused by the absorption of energetic ultraviolet and X - Ray radiation from the Sun.
Exosphere
It is the region above about 500 km. It possesses mainly oxygen and hydrogen atoms.

Fig: Layers of the atmosphere
Ozone layer
Ozone is found in the stratosphere. It acts as a shield in the atmosphere and absorbs radiation like ultraviolet rays from the sun. But the concentration of the ozone in the stratosphere changes with seasons. It is highest normally in the spring season, that is during the period from February - April. It is lowest during the July - October period. The absorption of UV radiation by the ozone layer increases with its thickness.
The thickness of the ozone layer is 0.29 cm above the equator and may exceed 0.40 cm above the poles during the winter season.

Fig: Ozone Layer
UV or ultraviolet rays
Ultraviolet (UV) radiation is a form of non-ionizing radiation emitted by the sun. Non-ionizing radiation is a type of low-energy radiation that does not have enough energy to remove an electron from an atom or molecule. UV rays are highly dangerous to living organisms. These radiations can damage DNA and proteins of living organisms. These molecules preferentially absorb UV rays, and the high energy present breaks the chemical bonds within these molecules. UV radiations are of 3 types as follows:
- UV-A
- UV-B
- UV-C
UV-A
It is also called the blacklight. It has a wavelength range between 320 - 400nm. It is the longwave radiation and is not very harmful. It causes ageing and wrinkling of skin. UV-A penetrates deeply into the dermis, reaching well into the dermis, and can damage DNA, which may lead to mutations.
UV-B
It has a wavelength range between 280 - 320nm. It is the intermediate wave radiation and is highly harmful. UV-B is almost completely absorbed by the epidermis, with comparatively little reaching the dermis. It causes ageing of skin, damage to skin cells and various types of skin cancers. In the human eye, the cornea is able to absorb UV-B radiation. In high doses it can cause inflammation of the cornea which is called snow-blindness. Such exposure may permanently damage the cornea. Snow blindness is a temporary eye pain and discomfort that happens due to ultraviolet (UV) light.
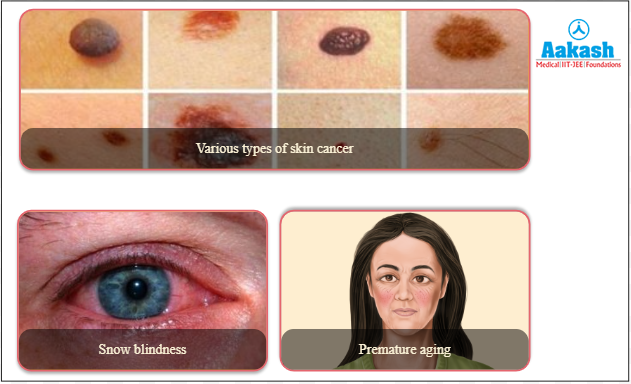
Fig: Effect of UV rays
UV-C
It has a wavelength of 200 - 290nm. It is the short wave radiation. UV-C radiation is almost completely absorbed by Earth’s atmosphere, given that the ozone layer is intact.
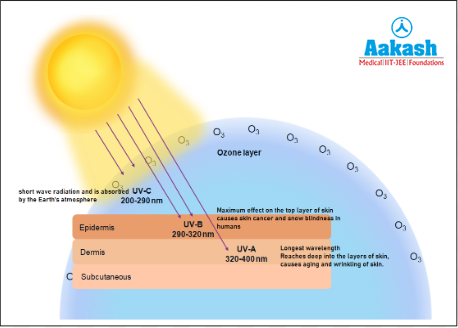
Fig: Ultraviolet rays
Dobson Unit
It is the unit of measurement of ozone in a column of air from the ground to the top of the atmosphere. The average thickness of ozone is 300 DU. 1 DU is equal to 1ppb. Parts per billion (ppb) is the number of units of mass of a particle per 1000 million units of total mass.
Fig: Dobson Unit
Formation of ozone layer
In the first step of ozone layer formation, UV rays from the sun break down molecular oxygen to form 2 oxygen atoms.
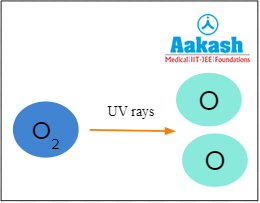
Fig: Formation of oxygen atoms
In the next step, oxygen atoms combine with molecular oxygen to form ozone, again in the presence of UV. There is a balance between production and degradation of ozone in the stratosphere. This is necessary to maintain the ozone layer.
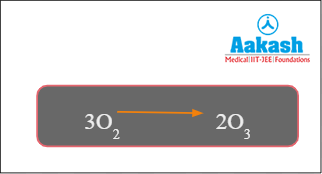
Fig: Formation of ozone
Depletion of ozone layer
The balance between production and degradation of ozone in the stratosphere has been disrupted due to enhancement of ozone degradation by chlorofluorocarbons (CFCs) which are used as refrigerants. CFCs discharged in the lower part of the atmosphere, move upward and reach the stratosphere. In the stratosphere, UV rays act on them releasing Cl (Chlorine) atoms. Cl acts as a catalyst (Cl atoms are not consumed in the reaction) and degrades ozone, releasing molecular oxygen. Here, the backward reaction becomes greater than the forward reaction. Since CFCs act as catalysts, they do not get consumed in the reaction. Hence, whatever CFCs are added to the stratosphere, they have permanent and continuing effects on ozone layer depletion.
A single chlorine atom can convert 1 lakh molecules of ozone into oxygen as it acts as a catalyst but does not get consumed in the reaction. The reaction is as follows:
UV-C
CFCl3 → CFCl2 + Cl
UV-C
CFCl2 → CFCl + Cl
Cl + O3 → ClO + O2
ClO + O3 → Cl + 2O2

Fig: Depletion of ozone layer
Ozone hole
Ozone hole is the area above Antarctica (shown in purple colour) where the ozone layer is the thinnest. The ozone hole present above Antarctica develops every year normally between late August and early October.
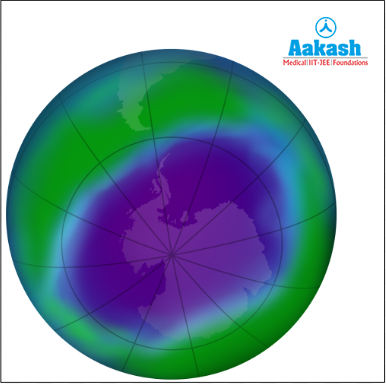
Fig: Ozone hole
Formation of ozone hole
Ozone hole was first discovered in 1985 by the Scientist Jonathan Shanklin. Ozone depleting substances released by industrialised countries of Europe, North America, Japan and Russia reach the stratosphere. From here they are pushed towards poles by winds. During winter there is no sunlight and the temperature is very low (-85°C). This favours the formation of ice clouds.
At this time the Antarctic air is completely isolated from the rest of the Earth. It circulates over the polar region and is called the polar vortex. These ice clouds provide the catalytic surface for chlorine. Here the chlorine and other reactants of ozone depleting substances react with ozone and degrade it.
Sunlight is required for this chemical process and it is available during the spring season. So more ozone depletion occurs in the spring season. This results in the appearance of an ozone hole. Ozone hole disappears normally during the summer as warmth mixes with the Antarctic air with air of other parts of the world, now the Antarctic air is not isolated.
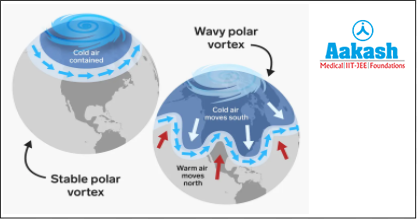
Fig: Polar vortex
Montreal protocol
After understanding the deleterious effects of ozone depletion, an international treaty was signed at Montreal in Canada in 1987. It is called the Montreal Protocol. It has been effective since 1989 to control the emission of ozone depleting substances by many countries. It was signed by 27 industrialised countries.
Kyoto Protocol
Kyoto Protocol is an international treaty that aims to reduce the emission of gases that contribute to global warming. The Kyoto Protocol was proposed at an International Conference on Climate change held in Kyoto, Japan in December, 1997. This protocol insisted that countries take appropriate measures to mitigate climate change. It insisted on reducing their overall greenhouse emission to a level at least 5% below the 1990 level.
The targets for the Kyoto Protocol was to control emissions of the six main greenhouse gases, namely: carbon dioxide (CO2); methane (CH4); nitrous oxide (N2O); hydrofluorocarbons (HFCs); perfluorocarbons (PFCs); and sulphur hexafluoride (SF6). Subsequently, many more efforts have been made and protocols have laid down definite roadmaps, separately for developed and developing countries, for reducing the emission of CFCs and other ozone depleting chemicals.
Earth Summit
The United Nations Conference on Environment and Development (UNCED) conducted the Earth Summit at Rio de Janeiro, Brazil in 1992. It aimed at reducing the greenhouse gas emission.
Practice Problems
Q1. Identify the ones which are not correctly described.
|
Present in stratosphere |
Measured in Dobson units |
|
320 - 400 nm wavelength |
Penetrates into dermis of skin |
|
290 - 320 nm wavelength |
Penetrates into subcutaneous tissues |
|
400 - 490 nm wavelength |
Partially absorbed by an intact ozone layer |
|
Used as refrigerants |
Catalyse the break up of ozone to oxygen |
A. 1 and 4
B. 2 and 3
C. 3 and 4
D. 4 and 5
Solution: The ozone layer is a thin part of the Earth's atmosphere that absorbs almost all of the sun's harmful ultraviolet light. It is present in the upper part of the atmosphere which is called the ‘stratosphere’. It acts as a shield absorbing ultraviolet radiation from the sun. The Dobson Unit (DU) is the unit of measurement of ozone in a column of air from the ground to the top of the atmosphere. The average thickness of ozone is 300 DU.
UV radiations are of 3 types viz. UV-A, UV-B and UV-C. UV - C (200 - 290 nm wavelength) radiations are almost completely absorbed by Earth’s atmosphere, given that the ozone layer is intact. But, UV - A (320 - 400 nm wavelength) and UV - B (290 - 320 nm wavelength) enter the epidermis and dermis layer of the skin respectively and damage DNA which may lead to mutations. However they do not penetrate into subcutaneous tissues. Thus rows 3 and 4 are incorrect descriptions of the UV rays.
Ozone layer is damaged by chemicals called Chlorofluorocarbons (CFCs), which are used in the manufacture of aerosol sprays, as blowing agents for foams and packing materials, as solvents, and as refrigerants. CFCs or chlorofluorocarbons discharged in the lower part of the atmosphere normally move upward and are able to reach the stratosphere. In the stratosphere, UV rays act on them releasing chlorine atoms. Chlorine acts as a catalyst (Cl atoms are not consumed in the reaction) and degrades ozone, releasing molecular oxygen. Hence option c is correct.
Q2. Match column I with column II and select the correct option.
|
Column I |
Column II |
||
|
1. |
Earth Summit |
P. |
Thinning of ozone layer |
|
2. |
Kyoto Protocol |
Q. |
Reducing emission of greenhouse gases |
|
3. |
Montreal Protocol |
R. |
Conservation of biodiversity |
|
4. |
Ozone hole |
S. |
Protect stratospheric ozone |
A. 1 - Q, 2 - R, 3 - S, 4 - P
B. 1 - Q, 2 - R, 3 - P, 4 - S
C. 1 - R, 2 - Q, 3 - S, 4 - P
D. 1 - Q, 2 - S, 3 - R, 4 - P
Solution: Ozone layer is found in the upper part of the atmosphere called the stratosphere. This layer acts as a shield by absorbing the ultraviolet radiation from the sun. The thinning of the stratospheric ozone layer is called the ozone hole. Ozone holes are found in the region of Antarctica. CFCs are the main pollutants that cause the depletion of the ozone layer. In 1987 an international treaty, known as the Montreal Protocol, was signed to control the emission of ozone depleting substances. However, it became effective from 1989. Kyoto Protocol (1997) is an international treaty aimed to reduce the emission of greenhouse gases that contribute to global warming. The UNCED (United Nations Conference on Environment and Development) (UNCED) was held in Rio de Janeiro, Brazil in 1992. It is called the Earth Summit. The main aim was to take appropriate measures for the conservation of biodiversity and sustainable utilisation of its benefits. Hence option c is correct.
Q3. Read the following statements (A - D) related to the formation of ozone in the stratosphere and arrange them in the correct order.
A. Ozone gas is formed.
B. 2 oxygen atoms are formed.
C. Oxygen atoms combine with molecular oxygen.
D. Ultraviolet rays of the sun will breakdown molecular oxygen.
a. A → B → D → C
b. D → A → C → B
c. D → B → C → A
d. B → C → A → D
Solution: Ozone is a molecule made up of three oxygen atoms, often referred to as O3. The high concentration of ozone that is found in the stratosphere around 15 - 30 km above the Earth's surface is called the ozone layer. It acts as a shield by absorbing ultraviolet radiation from the sun. The stratospheric ozone is formed naturally. It involves the chemical reactions between ultraviolet radiation and oxygen molecules.
The steps are as follows:
- Ultraviolet radiation breaks down molecular oxygen (O2).
- Two atoms of oxygen are formed.
- Each of these highly reactive oxygen atoms combine with an oxygen molecule forming ozone.
- Thus, ozone (O3) is formed in the presence of ultraviolet radiation in the stratosphere.
Ozone formation occurs continuously whenever solar ultraviolet radiation is present in the stratosphere. The production and destruction of stratospheric ozone is balanced. It is balanced through chemical reactions. Hence option c is correct.
Q4. Read the following statements (A - D) related to the depletion of the ozone layer by CFCs and select the correct option stating true (T) and false (F).
A. CFCs are the most damaging agents of the ozone layer.
B. Chlorine atoms are released due to the activity of UV radiation on CFCs.|
C. The active chlorine converts ozone into hydrogen atoms.
D. Cl atoms are consumed in the process of degradation of ozone.
a. A - T, B - F, C - T, D - T
b. A - F, B - T, C - T, D - F
c. A - T, B - T, C - F, D - F
d. A - F, B - F, C - T, D - T
Solution: The ozone layer present in the stratosphere, acts as a shield as it absorbs the harmful UV radiation and prevents it from reaching the Earth's surface. Unfortunately, the ozone layer is getting depleted due to the pollutants such as chlorofluorocarbons (CFCs) released into the atmosphere due to human activities. Ozone hole is the thinning of the stratospheric ozone layer which is clearly visible in the region of Antarctica. CFCs are commonly called ozone depleting substances. Ozone gas is continuously formed and degraded in the atmosphere by the action of UV rays but the balance is lost due to the presence of ozone depleting substances. CFCs are the most damaging agents of the ozone layer. They are widely used in refrigerants. CFCs discharged in the lower part of the atmosphere move upwards to the stratosphere. Here, they are acted upon by the UV radiation and free Cl atoms are released. The active chlorine atoms are able to destroy ozone catalytically by converting it into molecular oxygen. Thus, chlorine acts as a catalyst but does not get consumed in the reaction. Hence option c is correct.
Q5. What is the Kyoto Protocol, and what was its target?
Answer: Kyoto Protocol is an international treaty that aims to reduce the emission of gases that contribute to global warming. The main targets for the Kyoto Protocol was to control emissions of the main greenhouse gases. There are six main greenhouse gases. They are carbon dioxide (CO2); hydrofluorocarbons (HFCs); methane (CH4); nitrous oxide (N2O); perfluorocarbons (PFCs); and sulphur hexafluoride (SF6).
Q6. What is an Ozone hole?
Answer: Ozone hole is the area above Antarctica where the ozone layer is the thinnest. It develops over Antarctica every year between late August and early October.
FAQs
Question 1. Who discovered the ozone layer?
Answer: Charles Fabry and Henri Buisson discovered the ozone layer in 1913.
Question 2. Can we consider ozone as gas?
Answer: Ozone can be considered as a gas. It is composed of three atoms of oxygen. Ozone is present in the upper atmosphere of Earth and at ground level also. It can be good or bad, depending on where it is present.
Question 3. Differentiate between good ozone and bad ozone?
Answer: Stratospheric ozone is considered good ozone. It protects living beings from the ultraviolet radiation of the sun. The ozone present in the ground level is considered as bad ozone. It can trigger a variety of health issues, mainly for children, the elderly and the people with lung diseases such as asthma.
Question 4. Can we smell ozone?
Answer: Ozone has a characteristic smell which humans can detect even in small concentrations.
YOUTUBE LINK: Need to be created
Related Topics
|
Solid wastes, Practice Problems and FAQs |
|
Greenhouse effect and global warming, Practice problems, FAQs |
|
What is pollution,Types of pollution, Practice Problems and FAQs |
|
Air pollution and its control: Air pollutants, Effects of air pollution, Smog, Acid rain, Bhopal Gas Tragedy, Control of air pollution, Prevention of air pollution, Practice Problems and FAQs |
|
Deforestation, Practice Problems and FAQs |


