-
Call Now
1800-102-2727
Oxygen- Definition, Structure, Allotropes, Preparation, Physical and Chemical Properties, Anomalous properties & Uses
How long can you hold your breath? Well, for me it's not any more than 10 seconds! So what is it that we inhale through our nostrils every other second? It is a gas! But which gas is it, that is making us thrive on this beautiful planet of ours? Well! It's the omnipresent Oxygen!

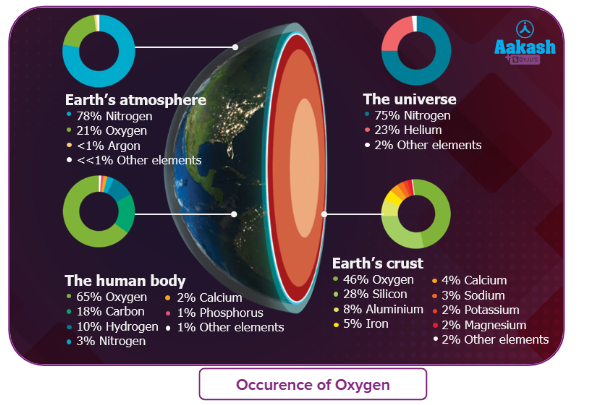
Oxygen is one of the most significant components present in the atmosphere. In its gaseous diatomic form, this element is essential for the survival of living organisms on the planet. Oxygen has an atomic number of 8 and belongs to group 16, period 2 of the modern periodic table. It is the first member of the chalcogen family. 21% of the earth’s atmosphere is occupied by oxygen, and more than half of the earth’s crust contains metals in their oxide forms.

Combustion of fuels in spacecraft and rocket launching also requires oxygen. Following hydrogen and helium, oxygen is the third most abundant element. Oxygen has large-scale applications right from industries to cellular respiration in biological systems. Plants play an important role in maintaining oxygen saturation in the atmosphere; it also helps in decreasing the carbon dioxide content in the air.
TABLE OF CONTENTS
- Definition of Oxygen
- Structure of Oxygen
- Allotropes of Oxygen
- Laboratory Preparation of Oxygen
- Industrial Production of Oxygen
- Physical Properties of Oxygen
- Chemical Properties of Oxygen
- Anomalous Properties of Oxygen
- Uses of Oxygen
- Practice Problems
- Frequently Asked Questions- FAQs
Definition of Oxygen
Dioxygen or popularly called as oxygen, is one of the most abundant allotropes of elemental oxygen and is represented by the chemical formula O2. It is also sometimes referred to as oxygen gas, molecular gas or dioxygen.
One of the most prevalent allotropes of elemental oxygen, dioxygen, sometimes known as oxygen, is denoted by the chemical formula O2. It can also be referred to as dioxygen, oxygen gas, or molecular gas.
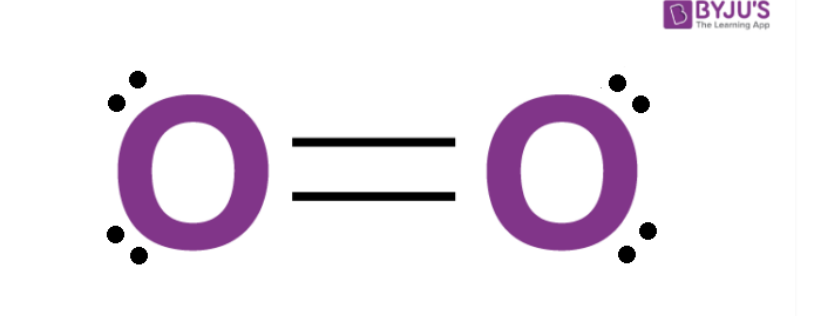
Structure of Oxygen
The Lewis structure of oxygen is represented by two double bonds between two oxygen atoms. Hence O2 molecule is linear, and it has strong electronegativity and reactivity due to lone pairs of electrons present on both the oxygen atoms. The molecule of oxygen can be represented as O=O, and the bond between the two atoms is covalent. The bond length of O2 molecule is 121 pm, whereas it's its bond energy is 498 kJ mol-1.
Allotropes of Oxygen
The most common allotrope of elemental oxygen is dioxygen (O2).
The next major allotrope is Trioxygen (O3) commonly known as ozone which is a very reactive allotrope of oxygen. Ozone is produced in the upper atmosphere when O2 combines with atomic oxygen made by the splitting of O2 by ultraviolet (UV) radiation.
Laboratory Preparation of Oxygen
- The thermal decomposition of peroxides such as hydrogen peroxide is used to produce oxygen. Commercially, barium peroxide has been widely used for this purpose.
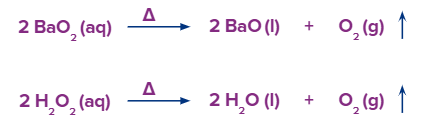
- The collection of oxygen is done by downward displacement of water. Oxygen is less dense than water and also barely soluble in water. Hence it is collected over water as there is no fear of excessive dilution.
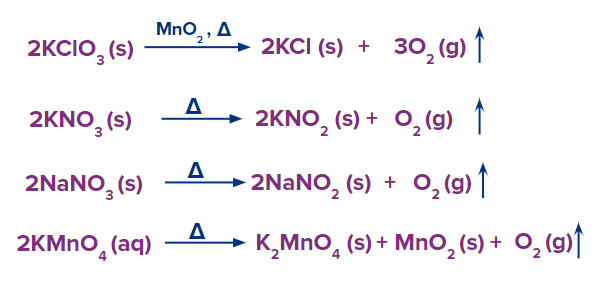
- Salts that are rich in oxygen, such as nitrates, chlorates and permanganates liberate oxygen on thermal decomposition. MnO2 is used as a catalyst to speed up the decomposition of chlorates of sodium and potassium at 420K.
- Several other metal oxides like mercury, silver, and lead whose electrode potential is low and are present lower in the electrochemical series, decompose to produce oxygen gas.
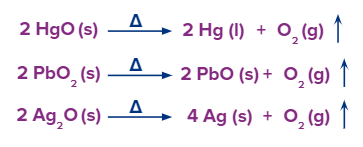
- Electrolysis of acidified water also produces 2 volumes of hydrogen gas and 1 volume of oxygen gas.
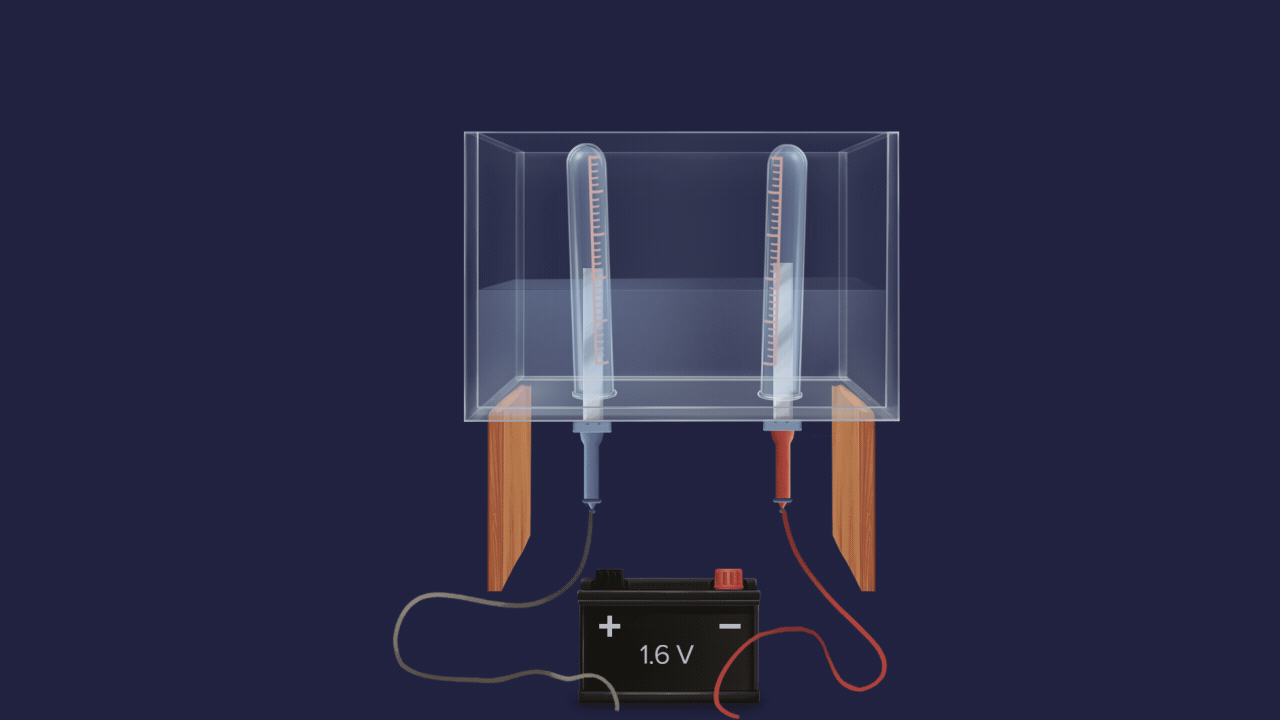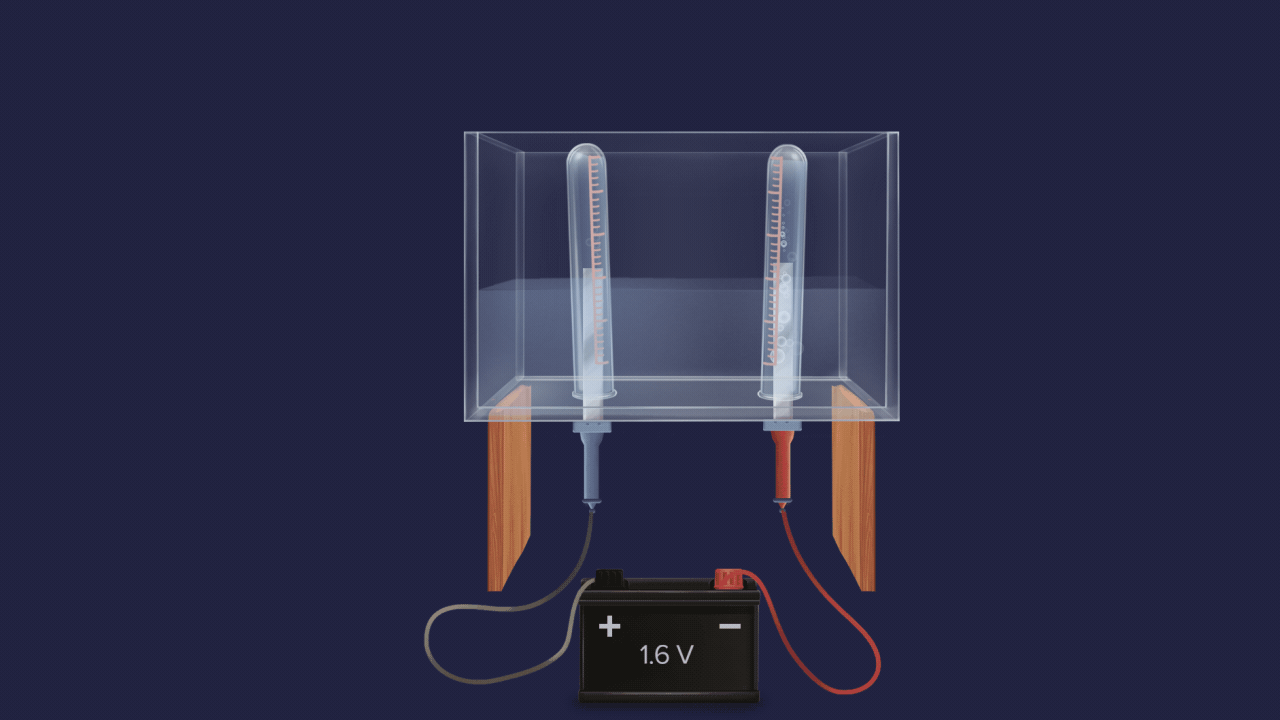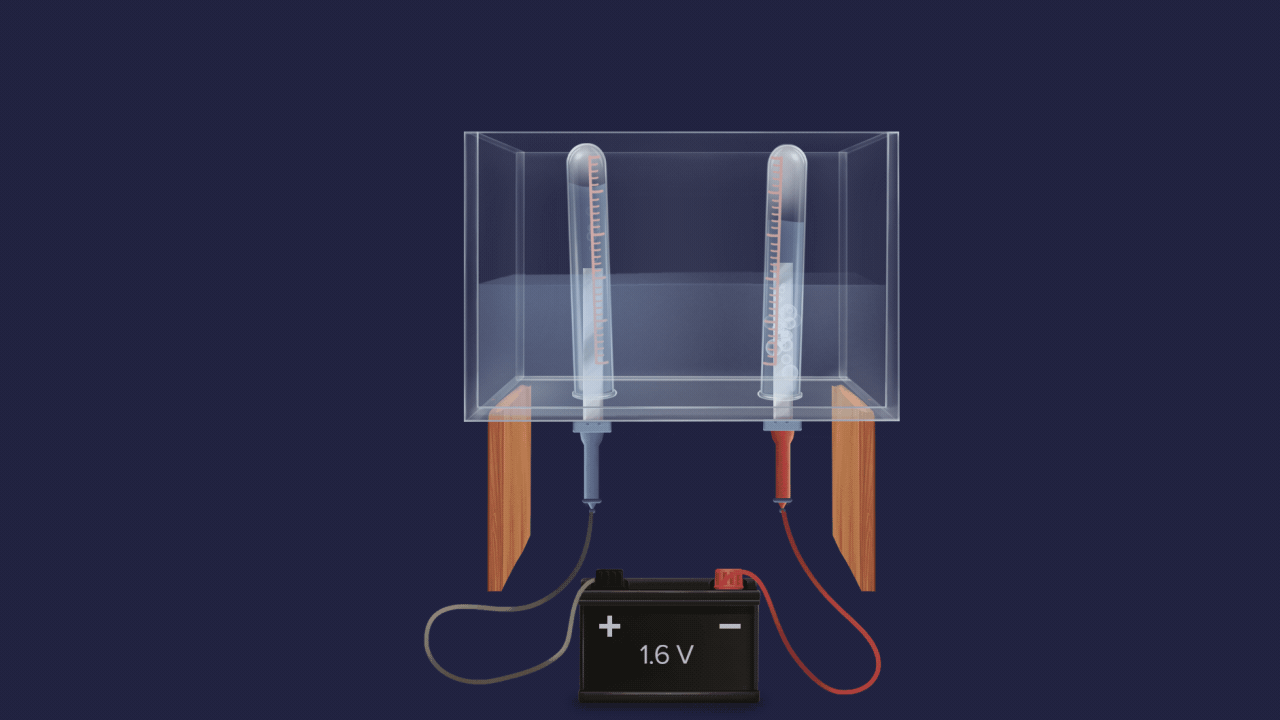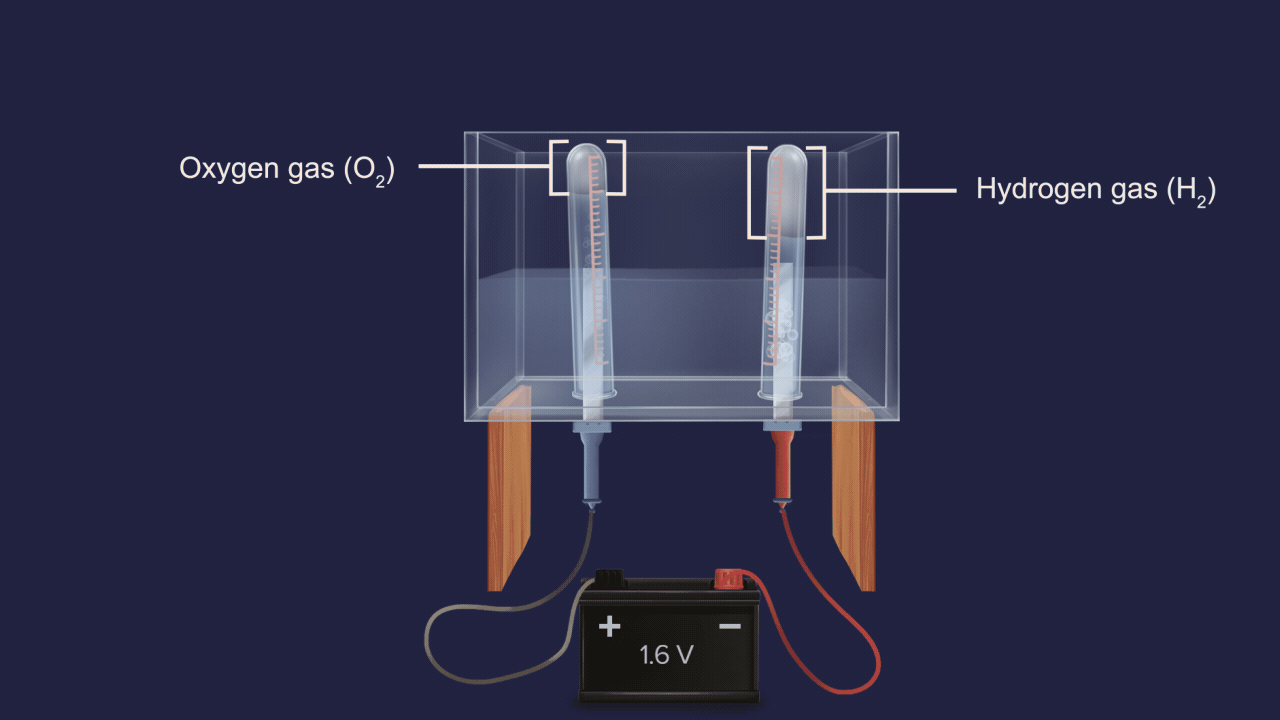
![]()
Industrial Production of Oxygen
There are primarily two methods of producing oxygen gas industrially.
- Firstly by fractional distillation of liquefied air. Nitrogen which constitutes 78% of air has a lower boiling point and hence it boils out first leaving only pure oxygen. The boiling point of oxygen, nitrogen and argon whose boiling points are: -183oC, -196oC, -186oC. After dust and other solid particles removal After the removal of dust and other solid particles, filtered air is cooled to –200°C. At this temperature, it is called liquified air. The liquefied air is then passed into the bottom of a fractionating column. First water vapour condenses and is removed using absorbent filters. Oxygen liquefies at -183oC and is extracted out. Nitrogen liquefies at -196oC and got extracted from the air.
- Another method is adsorption, by passing air through zeolite molecular sieves which absorb nitrogen and we obtain 90-93% pure oxygen.
Physical properties of Oxygen
- Oxygen is a colourless, odourless, tasteless diatomic gas and is paramagnetic in nature.
- Oxygen gas is heavier than air with a density of 1.429 gL-1.
- It is slightly soluble in water but soluble to such an extent that aquatic life can survive in the water. The solubility of oxygen is highly temperature dependent and decreases with an increase in temperatures.
- The atomic mass of oxygen is 8 u and the molar mass of O2 is 32 g mol-1.
- The melting point of oxygen gas is -218.4 ˚C, whereas the boiling point is -183˚C.
- Oxygen gas in its pure form is non-inflammable, however, it is a supporter of combustion.
Chemical properties of Oxygen
- Oxygen gas can react with metals as well as non-metals and can form oxides, peroxides or superoxides.
(Lithium oxide)
(Sodium peroxide)
(Potassium superoxide)
(Carbon dioxide)
(Sulphur dioxide)
- Metallic oxides are basic in nature (Group 1, Group 2 metals) whereas generally non-metallic oxides are acidic in nature.
Examples: Na2O, CaO, BaO, CuO are basic oxides and are acidic oxides.
- Some metallic oxides exhibit a dual behaviour. They show characteristics of both acidic as well as basic oxides. Group 13 metals like aluminium and gallium combine with oxygen to form amphoteric oxides which have the capability to react with acids as well as bases.
- Some oxides are neutral as they neither react with base nor with acid. Example- CO, N2O, NO. .
- Oxygen can form peroxide () with some main group elements (Na, Ba, K, Cs, Rb) as well as with hydrogen. In peroxide form, it exists in -1 oxidation state.
- Some alkali metals like K, Cs, Rb can form superoxides () with oxygen. In peroxide form it exists in oxidation state. The reactions can be represented as:
- Oxygen is a strong oxidizing agent. So, in a redox reaction, the molecule of oxygen itself undergoes reduction.
In this molecule, oxygen changes its oxidation state from 0 in O2 to +2 in MgO so it undergoes reduction while oxidising Mg.
- Oxygen gas acts as an oxidant in supporting the combustion of hydrocarbons.
- Rusting of iron occurs due to the attack of oxygen and moisture on iron surfaces.
[Hydrated Iron Oxide]
Anomalous Properties of Oxygen
In the periodic table, oxygen belongs to Group 16. But owing to certain characteristic features of oxygen, it shows some exceptional properties unlike the other members of its family. In fact, the main reasons for the anomalous properties of oxygen are small atomic radii, high electronegativity and absence of vacant d-orbitals. The anomalous properties of oxygen are as follows:
- Oxygen exists in a diatomic state whereas others in the same group are polyatomic due to strong bonds which is not possible in the case of oxygen.
- Oxygen is a gas while others in the group are solid. This is due to the weak van der Waals forces in oxygen and its ability to form bonds.
- Oxygen shows oxidation states of -2 mostly except in OF2 where it shows +2 and -1 in peroxides. Fluorine is the most electronegative element and hence is able to bring out the positive oxidation state of oxygen.
- Oxygen cannot extend its oxidation state above that unlike other members of its group which shows show -2, +2, +4, +6 oxidation states. For example- where sulphur is in +4, +6 and +6 oxidation states respectively. This is due to the absence of vacant d-orbital in oxygen.
- Oxygen gas is paramagnetic while others are diamagnetic.
- Hydride of oxygen, i.e., water is liquid whereas hydrides of other elements of group 16 are in gaseous form, this is due to its high electronegativity and tendency to form hydrogen bonding.
Uses of Oxygen
- In the biological system, oxygen is an integral part of cellular respiration that leads to the formation of ATP (adenosine triphosphate).
- Oxygen is used in the industries, in the manufacturing of glass, and also in mining.
- It is used in cutting and welding processes.
- In the steel industry, oxygen plays a crucial role in the smelting of iron.
- Oxygen reacts with ethylene across various stages to form ethylene glycol, a component of antifreeze agents and coolants.
- Oxygen is used in hospitals in case of medical emergencies.
- Oxygen is used as an oxidizer in rocket fuel.
- Oxygen is also used as a low-pressure gas in advanced astronaut suits to maintain the oxygen pressure and for proper ventilation.
Practice Problems
Q.1. Why does dioxygen exist as gas whereas sulphur exists as solid?
Answer: The O—O bond is comparatively weaker than the S—S bond owing to greater inter-electronic repulsions in the smaller sized smaller-sized oxygen atoms.
Also, due to smaller atomic radii and greater electronegativity of oxygen, it forms multiple bonds. As a result, molecular oxygen exists as O2 molecules held together by weak van der Waals forces. Hence, at room temperature, it exists as a gas.
Sulphur on the other hand has a lower tendency to form multiple bonds due to larger radii. Due to a larger atomic size, and low electronegativity, sulphur forms strong S—S single bonds, which explains its catenation property and so it exists as S8 molecules with a puckered-ring structure. As a result, sulphur is a solid due to a larger association, at room temperature.
Q.2. Does oxygen react with other elements of its own group?
Answer: Yes, it readily attracts electrons owing to high electronegativity and forms oxides with other elements of its group.
For Example
:
Q.3. Which of the following does not give oxygen on being heated?
A. HgO
B. KMnO4
C. K2Cr2O7
D. (NH4)2Cr2O7
Answer: (D)
Solution: All the given salts except ammonium dichromate liberate oxygen on heating. When ammonium dichromate is heated it decomposes to give off nitrogen gas and chromium oxide.
![]()
Q.4 Which of the following is not an acidic oxide?
A. SO2
B. CO2
C. Cl2O7
D. Fe2O3
Answer: (D)
Solution: Metallic oxides are basic and non-metallic oxides are generally acidic. Hence, iron oxide is the only option which is a metallic oxide and it is basic in nature.
Q.5 Find out in which of the following compounds oxidation state of oxygen would be zero? Which of the following compounds would have a zero oxidation state of oxygen?
A. HOF
B. HOBr
C. HOCl
D. HOI
Answer: (A)
Solution: Due to the highest electronegativity of fluorine, it always exists in -1 state and hydrogen exists as +1. Hence in hypofluorous acid (HOF) oxidation state of oxygen is zero. Only with fluorine, oxygen exhibits +1 and 0 oxidation states. Only with fluorine, does oxygen exhibit a +1 oxidation state.
Frequently Asked Questions-FAQs
Q.1. Does Oxygen react with alkali metals?
Answer: Oxygen has a high reactivity for electropositive elements and especially with alkali metals (Group I elements). It is recommended that alkali metals should be kept away from oxygen to avoid an explosion due to oxidation. Metals at the bottom of the group are more reactive than those at the top.
Q.2. Can oxygen exist as solid, liquid or gas?
Answer: At suitable temperature conditions oxygen can be obtained in all three states of matter. For example, solid oxygen can be obtained at normal atmospheric pressure at a temperature below 54.36 K (−218.79 °C). At room temperature oxygen exists as a gas. Solid and liquid oxygen is bluish in colour.
Q.3. Is oxygen available on mars?
Answer: According to the scientists of NASA, mars does have oxygen, but it is not sufficient enough to just roam around and breathe in fresh oxygen as we do on Earth. This is because the density of oxygen on Mars is times that of oxygen on Earth.
Q.4. Can oxygen ever become extinct from earth?
Answer: Few studies state that one billion years from now, the earth will have a very minimal amount of oxygen making it difficult for all aerobic processes to occur. But yet there is hope to reverse the process, only if we start following a sustainable process of livelihood.


