-
Call Now
1800-102-2727
Conformation- Formation and Definition of Conformation, Sawhorse Projection, Newman’s Projection, Conformers of Ethane, Conformers of Butane, Difference between Conformation and Configuration, Practice Problems and FAQs
Suppose you went to watch a movie. During the interval time of the movie movie, you might have gone outside for some refreshment. On returning obviously you occupy the same seat and complete watching the latter half of the movie. So what, you may ask? The question is that, why did you sit? You could have watched the movie by standing. What will change it make, whether you are sitting or standing? Let me tell you the answer to this question.
When you stand or sit, there is a change in the orientation of your body and there is a general tendency for a body to be in a state with more stability and rest.
All living and nonliving subjects like to experience a state of rest of the lowest energy. Living things attain it by sitting and laying down. Molecules experience several forces of attraction and repulsions arising from their neighbouring atoms, or groups. They too try to resist or reduce the forces of repulsion to having have the least energy. There are many ways by which the molecules carry out this. One such way is that single sigma-bonded carbon atoms do it by rotation around it. Let us know more about this rotation and the results.
Table of content
- Formation and Definition of conformers
- Sawhorse projection
- Newman’s projection Newman’s projection
- Conformers of ethane
- Conformers of butane
- Difference between conformation and configuration
- Practice problems
- Frequently asked questions-FAQ
Formation and Definition of conformers
Two carbon atoms in an organic compound may have a single covalent sigma bond only or in addition pi-bonds. The respective carbon atoms can easily rotate around the single sigma bond, while the presence of pi-bonds prevents it from doing so. The rotation of carbon atoms will take the attached atoms or groups along with them, resulting in different orientations with respect to others. These different positions of atoms or groups constitute different spatial positions for the same molecules. But each spatial position will experience different forces of interactions and hence shall possess different energy. Of course, the spatial orientation with least the least energy will be the most stable configuration for the molecule.
All the possible spatial arrangements of atoms due to the free rotation around C-C single bonds are called conformers or rotamers.
Characteristics of conformers:
- What will be the number of conformers? Rotation around 360° could result in an innumerable number of isomers. But we shall consider rotation around an incremental 60° and the resulting conformers or rotamers rotamers.
- The conformations around these rotation angles are referred as to as eclipsed, gauche, staggered and anti-conformational forms forms.
- Is the rotation total free? No. It to needs energy for rotation. But it is so small that the room temperature is sufficient to provide enough energy for rotation rotation.
- The different conformations are going to have different interactional energies called “torsional energy” which will restrict free rotation and the preferred conformation over others.
- Can we separate the conformers?
No. Isomers have fixed configurations and possess a larger difference in energy between them. Conformers on the other hand differ by a mere 1 to 10kJ 10 kJ of energy. Hence isomers can be separated, while it is very difficult to separate conformers from one another.
- Nevertheless, the stable conformers are useful in explaining the reaction mechanism mechanism.
Sawhorse Projection
In sawhorse a sawhorse projection, the wanted C-C molecular axis is viewed from sideways with each carbon atom on either end of the axis are attached to their respective atoms or groups at 120° angle. Sawhorse Projections can also be drawn in such a way that the groups on the front carbon are staggered (60 degrees apart) or eclipsed (directly overlapping) with the groups on the back carbon.
For example example, let us consider the two saw horse projection for ethane molecule
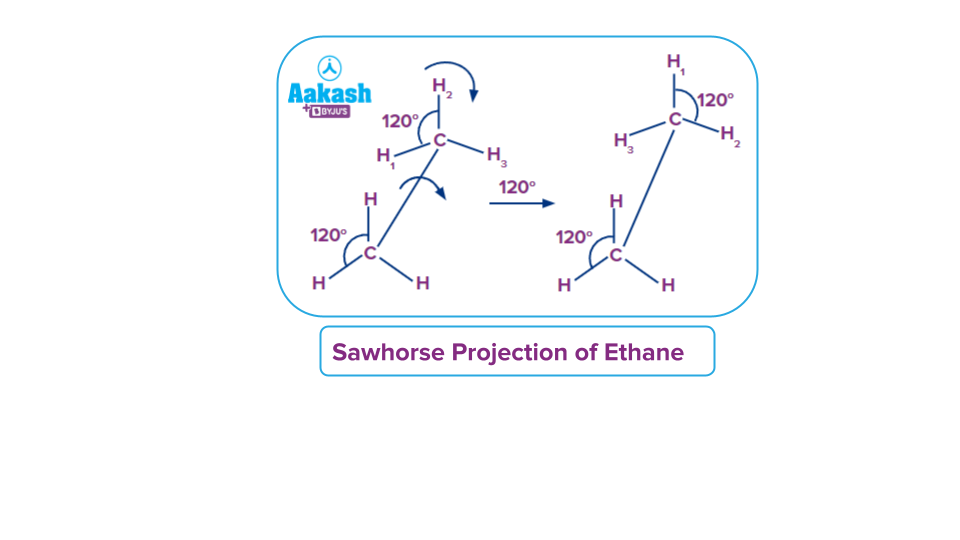
The upper end of the line is rotated towards the left or right-hand side along the carbon-carbon single bond. The front carbon present is attached with three hydrogen atoms similarly back carbon contains three hydrogen atoms respectively. Lines are inclined at an angle of 120 degrees to each other. We can rotate the carbon atom along the single bond at an angle of 120 degrees to obtain other sawhorse structures.
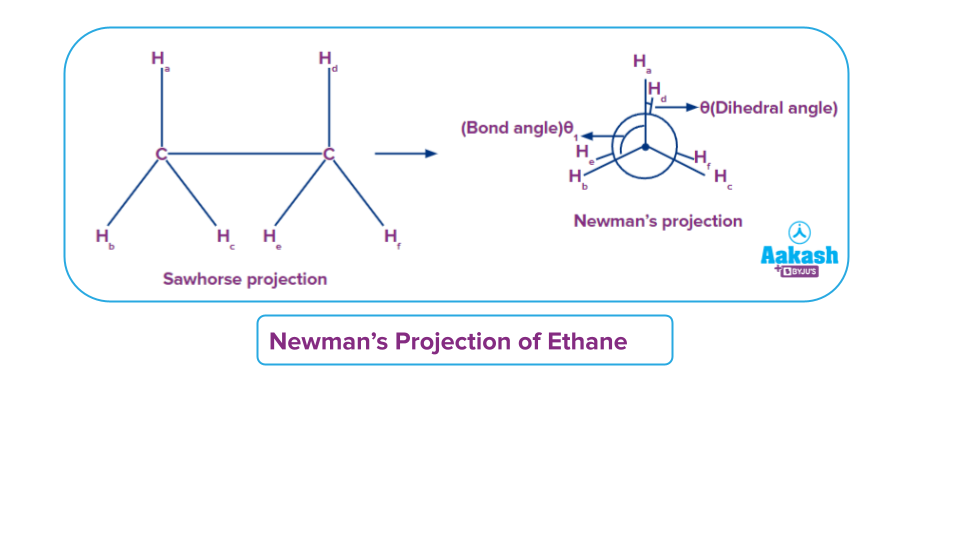
Newman’s Projection
In Newman’s projection the molecule is placed as a front and back model, with the C_C molecular axis directly in front, so that the first carbon and its attachment can be seen clearly. The second carbon and the bond connecting it to the first carbon will be invisible. Of course, the attachment to the back carbon can be seen in conformers other than eclipsed position. In the case of the back carbon. Newman projections are an easy visualization of conformers of rotation..
The three lines in the shape of a Y in a Newman projection represent the three bonds of the first carbon that are attached to three different valencies and the fourth valency of the front carbon cannot be seen as it is present behind the carbon atom connecting the back carbon atom. The back carbon is represented by a circle, and the three lines coming out of the circle represent the three bonds that come off of that carbon. It is important to note that the fourth bond for each of these carbons is the carbon-carbon bond which cannot be shown in the Newman projection. A Newman projection can aid in the analysis of rotation around a specific carbon-carbon bond.
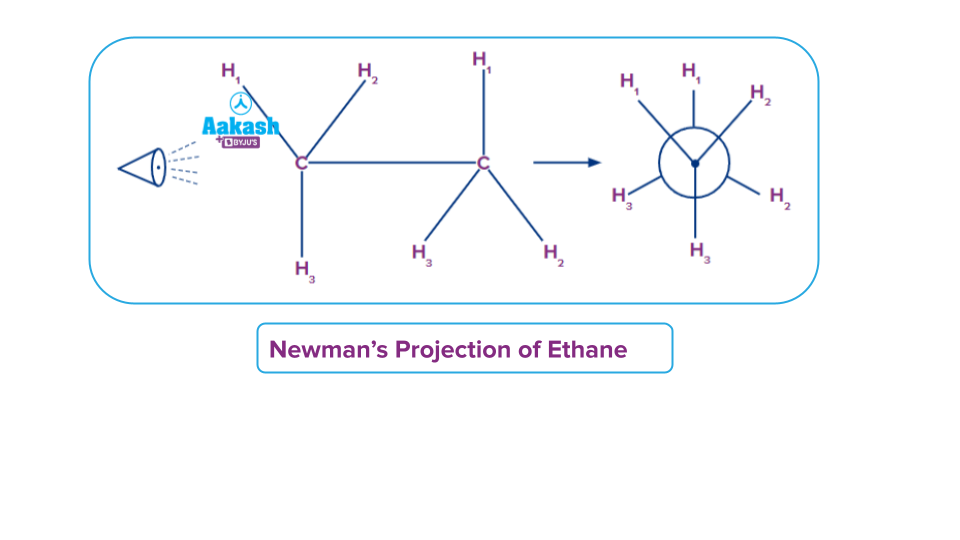
Same The same group will be attached as shown in the image given below.
When we view the molecule as shown, the front carbon is represented by the dot with its three valencies attached by solid lines and the fourth valency is present behind therefore it can’t be seen in Newman's projection. The 2nd carbon connected with the 1st carbon is represented by the larger circle with its three valencies attached to it as shown in the image.
Conformations of Ethane
Ethane(C2H6) Ethane (C2H6) contains a carbon-carbon single bond and each carbon atom is also attached to three H atoms. For understanding the concept let's take an example with Newman's projection model. Keep one carbon atom stationary and rotate other carbon atoms in a carbon-carbon bond. This rotation results in many spatial arrangements of an H atom attached to a carbon atom and another hydrogen attached to another carbon. This process is called conformational isomers.
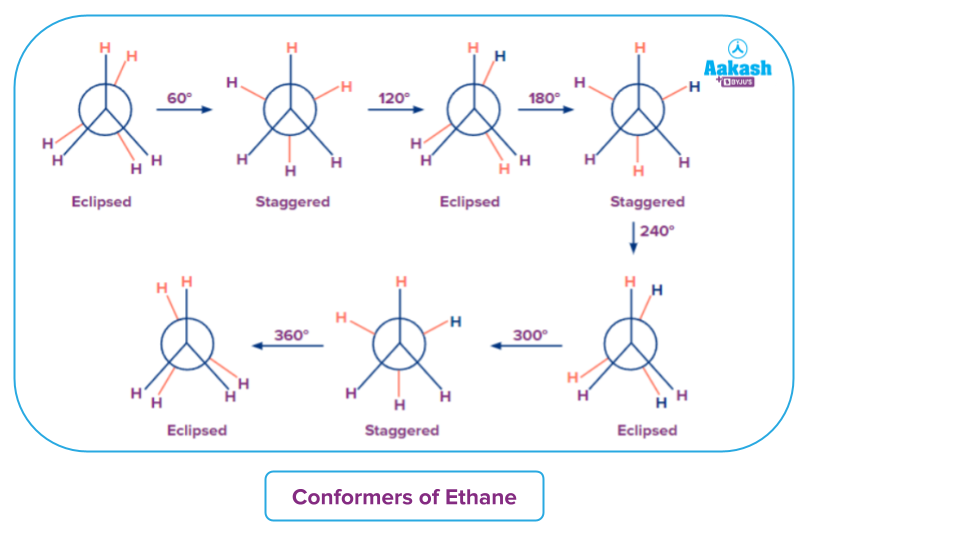
When one carbon is rotated along with the valency attached to it keeping the valency of other carbon atoms constant we obtain the different conformational isomers. When the angle between the front carbon atom and the back carbon atom is changed at a multiple of 60 degrees( until it is rotated to 360 degrees) two standard forms called eclipsed and staggered conformations will be obtained. Staggered and eclipsed forms are interposed between each other. The conformations in which hydrogen atoms attach to two carbon makes an angle of 0 degrees (i.e dihedral angle corresponds to 0 degrees) is called Eclipsed. The eclipsed conformation of ethane is less stable than staggered.
Staggered or anti - The conformation in which hydrogen atoms are at an angle of 180 degrees is called staggered or anti forms. The stable conformation of ethane is staggered conformation.
Skew or gauche - Intermediate conformation between two standard conformation conformations is called Skew or gauche conformers.
When the bond is rotated along the single bond to form different conformers dihedral angle which is represented by () changes but the bond angle between carbon and the attached hydrogen remains the same.
When the bond is rotated along the single bond to form different conformers, the dihedral angle () changes, but the bond angle between carbon and the attached hydrogen remains the same.
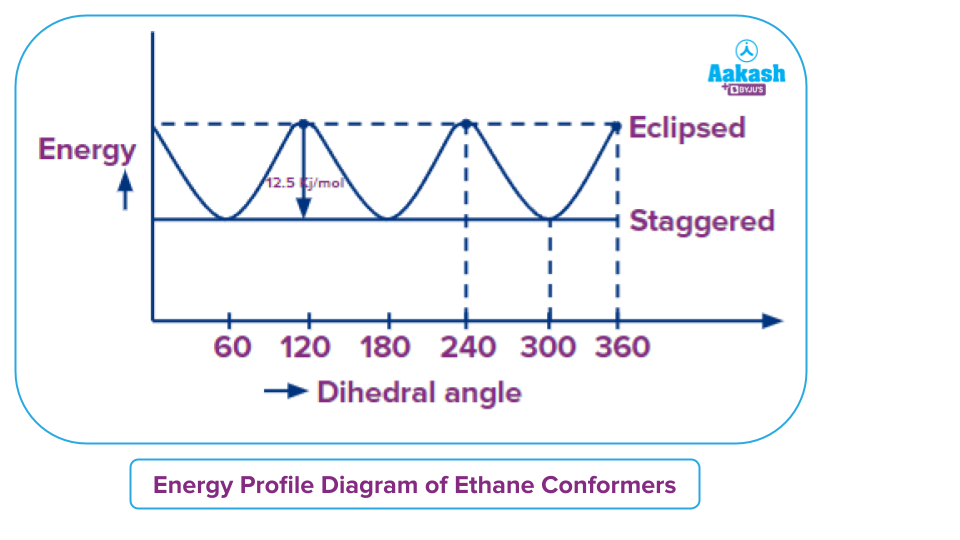
The relative energies of conformers can be plotted as shown.
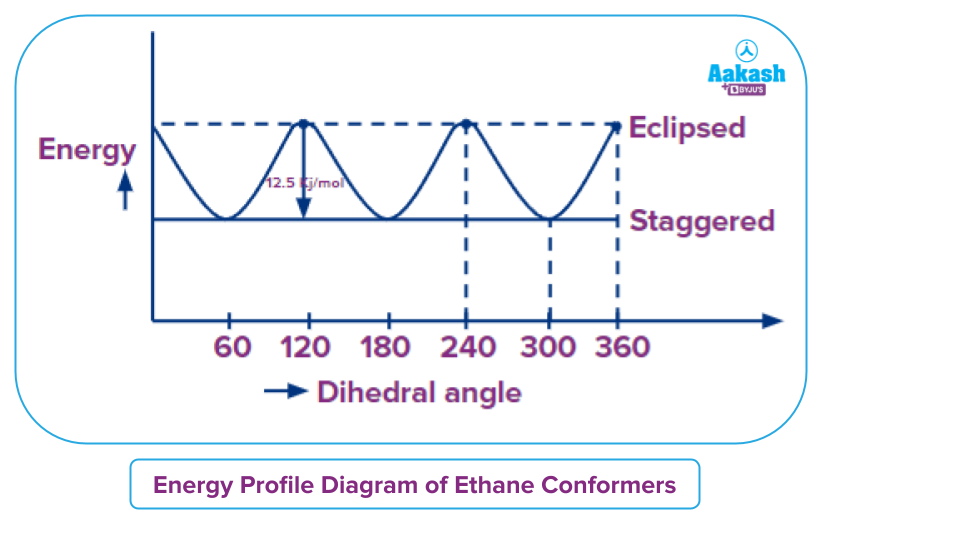
Eclipsed conformation can have two types of strains-
- Torsional: This is the repulsive force which is operated between the molecule due to bond-pair bond-pair repulsion between the adjacent atom attached to two different atoms. It operates in case of eclipsed conformation.
- Van der Waal strain: This strain is observed due to the steric hindrance of the bulky group and in the stable conformation bulky group tries to orient themselves to a maximum distance so that there is no steric hindrance or steric crowding in the conformation.
The most stable form of the ethane conformation is known as staggered conformation because both the torsional strain and Van der Waal strain will be absent. Whereas, in the case of the most unstable form of the ethane conformation, the eclipsed form there is a torsional strain which is present due to bond pair bond pair bond pair-bond pair repulsion as dihedral angle corresponds to 0 degree. But the Van der Waal’s strain will not be observed because the size of the hydrogen atom is small and the difference in the energy of most the most unstable conformation and most the most stable conformation corresponds to the value corresponds to 12.5 Kj mol -1. This energy is also called energy the energy barrier which is generally available at room temperature for rotation of the molecule.
Conformers of butane molecule
Consider the butane molecule along the (along C2-C3) bond. Two hydrogens and one methyl group will be attached to the front carbon(shown in solid lines) and the 4th valency cannot be seen as it is behind the 1st carbon atom. In the case of the back carbon ( shown by a circle), the same groups will be attached as shown in the image given below.
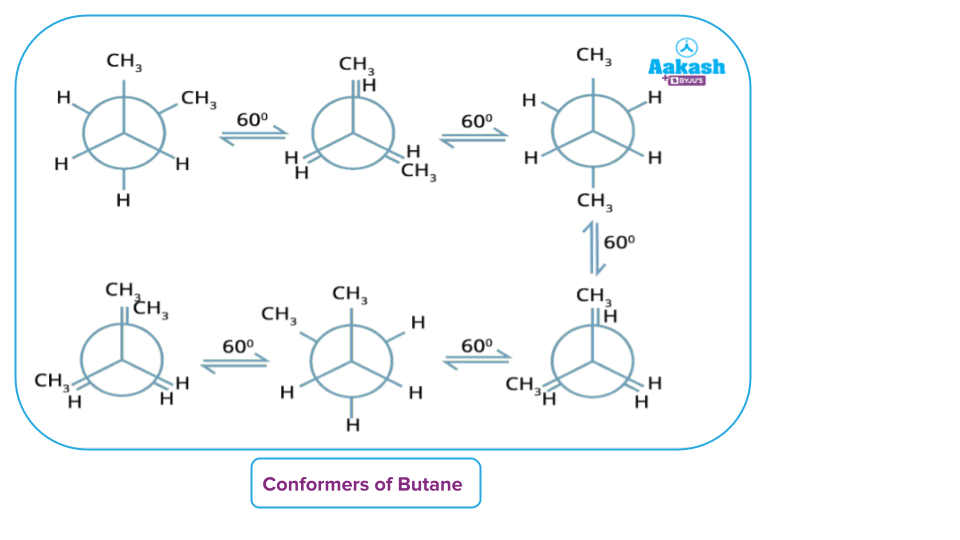
When the methyl group attached to the back carbon is just behind the methyl group of front the front carbon such that the dihedral angle corresponds to 0 degrees it is said to be in eclipsed conformation. When the group is rotated at an interval of 60 degrees the six different conformers are obtained. When the dihedral angle corresponds to 60 degrees the obtained form is known as gauche form, at 120 degrees it is known as partially eclipsed form, at an angle of 180 degrees it is said to be in staggered form. On further rotation at an angle of 240 degrees, 300 degrees and 360 degrees the form obtained is known as partially eclipsed, gauche and eclipsed conformation
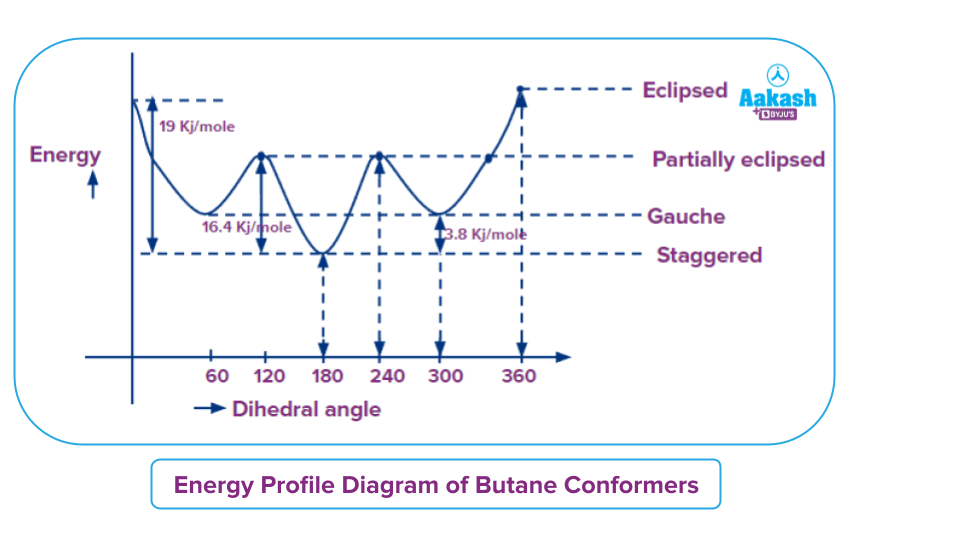
The most stable form of the butane conformation is known as staggered conformation because both the torsional strain and Van der Waal strain is absent. Whereas, in case of the most unstable form of the butane conformation is known as an eclipsed form in which there is a torsional strain which is present due to bond pair repulsion as dihedral angle corresponds to 0 degree as well as van der Waal strain because of two methyl group are present behind one other.
The gauche form is comparatively more stable than the partially eclipsed form and eclipsed form because in gauche form only a slight amount of Van der Waal strain is present but there is no torsional strain. Whereas in the case of partially eclipsed form torsional strain is present but there is no Van der Waal strain. The difference in the energy of most unstable conformation and most stable conformation corresponds to the value corresponding to 19 Kj mol -1 this energy is also called energy barrier which is generally available at room temperature for rotation of the molecule.
The order of stability of different conformers of butane are
Staggered > gauche > partially eclipsed > eclipsed
respectively.
Difference between conformation and configuration
|
S. No. |
Properties |
Conformation |
Configuration |
|
1 |
Definition |
The distinct arrangement of atoms in any molecule that can easily interconvert is known as conformation. |
The distinct arrangement of atoms in any molecule that cannot easily interconvert is referred to as its configuration. |
|
2 |
Interconversion |
Interconversion or converting one conformation into another (of the same molecule), is simple (takes place at room temperature). |
Interconversion, or converting one configuration to another, is difficult. |
|
3 |
Mechanism of Conversion |
Rotations around carbon to carbon single bonds is used to interconvert conformation. |
Breaking and forming new chemical bonds allows for configuration interconversion. |
|
4 |
Flexibility |
Conformations are highly adaptable. |
Configurations are less adaptable |
Practice problems
Q1. Which of the following strain is possible in the eclipsed form of ethane molecule?
A. Torsional strain|
B. Van der Waal strain
C. Only Van der Waal strain
D. Both A and B
Answer:(A)
Solution: The most stable form of the ethane conformation is known as staggered conformation because there is no torsional strain or Van der Waal strain. Whereas, in case of the most unstable form of the ethane conformation is known as an eclipsed form in which there is a torsional strain which is present due to bond pair bond pair bond pair-bond pair repulsion as dihedral angle corresponds to 0 degree, Van der Waal strain which is observed due to the bulkiness of the molecule but will not be observed in ethane molecule because the size of a hydrogen atom is small.
Q2. Which of the following forms may be obtained when the molecule is rotated along C2-C3 bonds present in the butane molecule?
A. Staggered
B. Gauche
C. Eclipsed
D. All of the above
Answer: (D)
Solution: All the three forms will be observed in the case of butane molecule when rotated at an angle multiple of 60 degrees. Eclipsed form corresponds to the most unstable form as both the methyl group is present behind one another in two different carbon followed by the gauche form in which two methyl group is at an angle of 60 degrees adjacent to each other. The staggered form is the most stable form as both the methyl group is at an angle of 180 degrees with respect to each other.
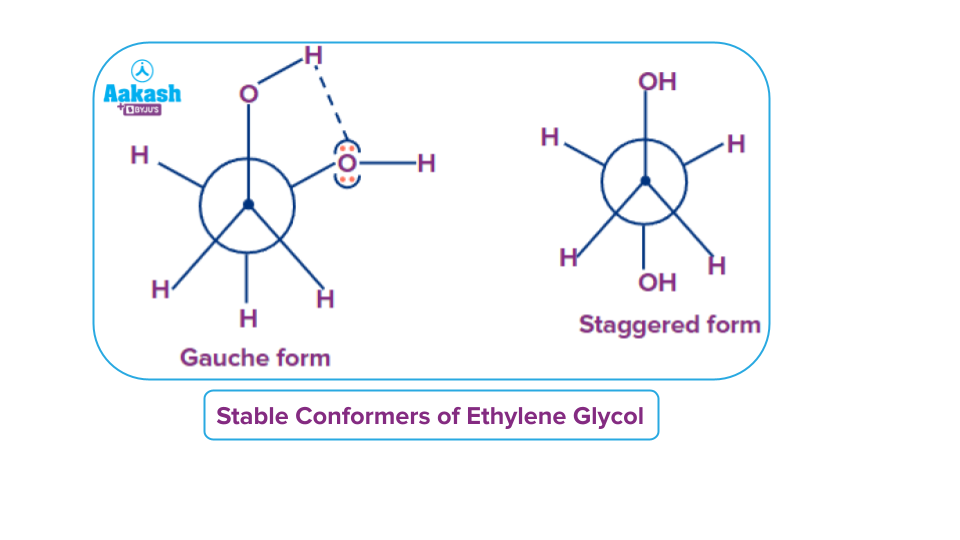
Q3. Select the most stable form of conformers when the rotation of C1-C2 bond takes place in ethylene glycol molecule.
A. Staggered
B. Gauche
C. Eclipsed
D. All will have the same stability
Answer: (B)
Solution: In general staggered form is more stable than gauche form but in the case of molecules like ethylene glycol which has intramolecular H-bonding in such case stability of gauch form is more than the staggered form.
In general, the staggered form is more stable than the gauche form, but in the case of molecules like ethylene glycol, which has intermolecular hydrogen bonding, the stability of the gauche form is more than that of the staggered form.
Q4. Select the correct option for the difference in the energy for a most stable and most unstable form of conformer in ethane molecule when rotated along (C1-C2) bond.
A. 12.5 Kj mol -1
B. 10.2 Kj mol -1
C. 9.5 Kj mol -1
D. 17.7 Kj mol -1
Answer:(A)
Solution: The most stable form the ethane conformation is known as staggered conformation because there is no torsional strain or Van der Waal strain is absent. Whereas, in case of the most unstable form of the ethane conformation is known as an eclipsed form in which there is a torsional strain which is present due to bond pair bond pair bond pair-bond pair repulsion as dihedral angle corresponds to 0 degree but van der Waal strain will not be observed because the size of a hydrogen atom is small. Due to this, there is a difference in the energy of the most stable form and the most unstable form and the value corresponds to 12.5 Kj mol -1
Frequently asked question-FAQ
Q1. How do we determine the relative stability of the conformers?
Answer: There are two types of strain present in the conformers Van der Waal strain and torsional strain torsional strain. In general van der Waal strain which is present due to the steric crowding of the group makes the conformation unstable and has higher energy than the conformer which exhibits torsional strain exhibited due to the bond-pair bond pair repulsion of the group attached on the front and back carbon. The relative stability of conformers is determined with the help of the type of strain present in it. Experimentally it is determined by the energy difference required to overcome the barrier.
Q2. What is a rotation restricted rotation-restricted system?
Answer: A rotation restricted rotation-restricted system is the type of orientation in which rotation of the bond is not possible along the atom attached to it. In the case of double and triple bonds bonds, pure the pure orbital participate in the bonding and it cannot be rotated until we break the bond.
Q3. What is the dihedral angle?
Answer: The dihedral angle is defined as the angle between two groups attached to two different carbon atoms in the Newman configuration. When the valencies along the carbon are rotated it leads to the change in the dihedral angle and therefore alters the stability of the conformer.
Q4. How to determine the stability of staggered and gauche conformation?
Answer: In general the staggered conformation is more stable than the gauche form because in the case of staggered form both torsional strain as well as the Van der Waal strain is not present as the dihedral angle corresponds to 180 degrees. But where intramolecular H-bonding is observed in such cases the stability of the gauche form is exceptionally more than the staggered form.


