-
Call Now
1800-102-2727
Methods of Separation-Handpicking, Threshing, Winnowing, Sieving, Magnetic separation, Filtration, Sedimentation, Separating Funnel, Solvent Extraction, Practice problems, and FAQs
Have you ever witnessed cotton picking? After picking cotton, it is processed through different types of machines and equipment to remove impurities from it. But even after all the processes, hair and white plastic could not be separated. To solve this, many textile companies, which are very much concerned about the quality of the product, employ manual labour to separate this impurity from cotton. This method is called hand picking, which is a very old technique of separation. Like the hand picking method there are many separation methods which are going to be discussed in this concept page.
Table of content:
- Handpicking
- Threshing
- Winnowing
- Sieving
- Magnetic separation
- Evaporation
- Distillation
- Filtration
- Sedimentation
- Separating funnel
- Decantation
- Sublimation
- Crystallisation
- Chromatography
- Solvent Extraction
- Practice Problems
- Frequently Asked Questions(FAQs)
Handpicking:
This process basically involves hand-picking out all the harmful components and separating them from the helpful ones. Both of the separated components might be helpful, or they might be impurities that need to be removed.
Hand-picking is a method used to remove undesired components from wheat, rice, and pulses, such as small pieces of stone.
Foodgrains with minor stone fragments are shipped in a flat container. One by one, hands remove the stones from the grains and discard them. All the stone bits are gone, and all that is left are the grains of food.

Threshing:
This technique is primarily applied when harvesting crops. Wheat stalks are often dried after harvest. The grain is removed from the stalks and ground into the floor by hammering the dry stalks to shake off the dried grains.
A food grain crop, like wheat or paddy, is harvested from the field once it has reached maturity. The crop is then dried in the sun. Bundles of dried agricultural plant stalks or stems that have grains connected at the top are sent to us.
The grains adhering to the stems or stalks are covered with a thin layer of chaff. Grain covered in chaff is tightly packed into each stalk. Chaff, stems, and stalks are removed from grains.
Grain and stems or stalks are separated during the threshing process. Beaten wheat or paddy stems are threshed to remove the grains from the stems and the chaff that covers them.
Holding bundles of stems in one's hands and threshing them against a hard surface is how threshing is done. The outcome is that the stems and grains separate.

Cattle are used to aid in threshing as well. A small area of the ground is covered with the harvested and dried agricultural plants, and various cattle, including buffaloes and camels, are made to graze over them for an extended period of time. The grains are separated from the stems by the cattle's foot crushing the stems or stalks. The chaff that surrounds the grains is broken by the crushing, enabling the separation of the grains from the chaff. The stalks are ground into hay, which is used as dry cow feed, during the threshing process. Broken chaff is used to make the husk. A motorised device called a thresher is used for the threshing process.
Winnowing:
A farmer gets a mixture of wheat grains and husk as he threshes his wheat crop. The husk of the wheat grain must be removed before it can be utilised. Winnowing is the process that separates the husk from the wheat grains.
Winnowing is the process of using the wind to separate grain husk from grain. Husk weighs less than wheat grains, which are heavier. A winnowing basket is used for the process of winnowing. The farmer repeatedly shakes his winnowing basket while standing on a platform that is higher above the ground, causing the mixture of wheat grains and husk to fall from a height.
Wheat grains form a pile on the ground when they fall vertically to the ground due to their weight. Because husk fragments are lighter, the wind may carry them farther. The outcome is that the husk separates from the wheat grain heap and forms its own heap. In this way, the husk is removed from the wheat grains.
Winnowing is a method for removing the husk from grains like rice and wheat.
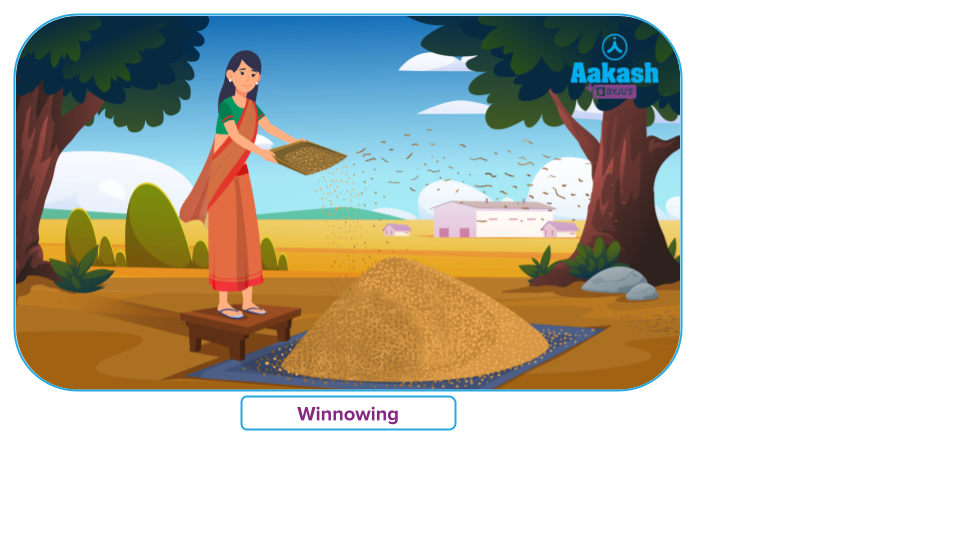
Winnowing cannot be used to extract small stone fragments from wheat. This is because stone fragments are relatively heavy and cannot be transported over great distances by wind.
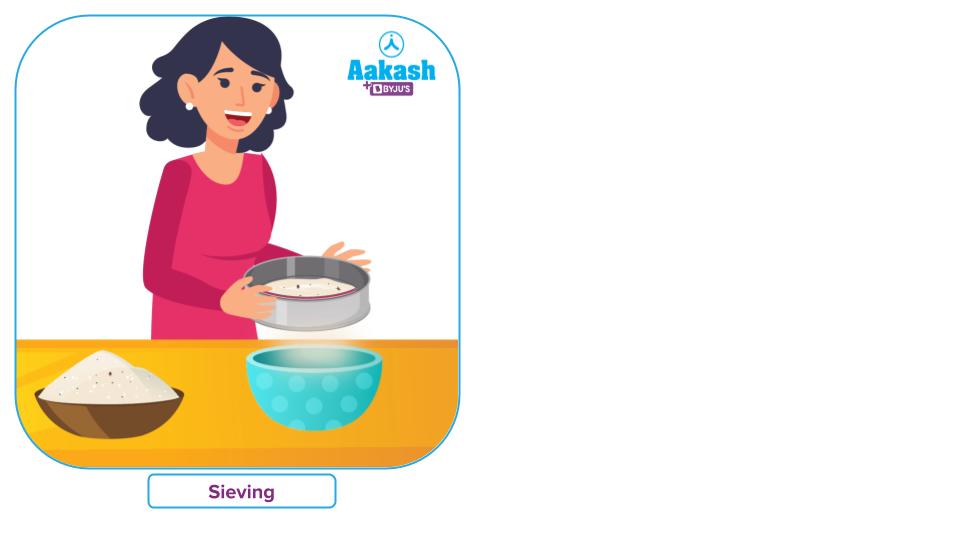
Sieving:
A shallow container called a sieve has tiny holes at the bottom. Sometimes a sieve can also be made of iron mesh. The act of sieving involves using a sieve to separate a mixture.
It is employed to separate mixtures of predominantly various-sized components.
Different-sized mixes are added to a sieve that is continuously tipped back and forth. The mixture's larger particles become stuck inside the sieve because they can't fit through the tiny holes.
Small and large particles separate out of the mixture to form two distinct parts. The size of the material's particles that need to be separated from the mixture determines the size of the sieve's holes. Various compounds are divided using sieves with varying hole sizes.
Example:
We sieve the wheat flour we get from a flour mill using a sieve with very small holes. Sieving flour is done to remove the coarse particles of flour from it so that fine flour can be obtained for making chapatis. When wheat flour is placed in a sieve and moved back and forth repeatedly, fine particles of flour pass through the sieve's small holes and collect in the vessel kept below. The larger particles of wheat bran do not pass through the sieve's very small holes and remain in the sieve. Wheat flour is separated from wheat bran and fine flour in this manner.
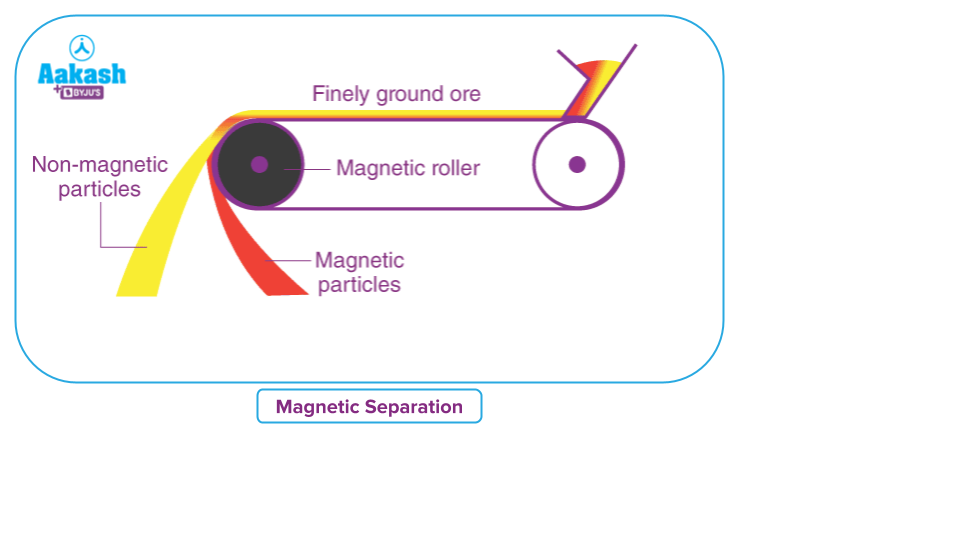
Magnetic Separation:
This method is very useful when one of the substances in the mixture has magnetic properties. To separate
magnetic elements, strong magnets are commonly used.
A magnet can be used to separate an iron filling and sulphur powder mixture. This is due to the fact that iron filling is magnetised, whereas sulphur is not.
A horseshoe type magnet is moved across the surface of the mixture to separate the iron filling from the sulphur. The magnet attracts the iron fillings, which cling to the magnet's poles and separate. To achieve complete separation of iron filling, this process must be repeated several times. Because the sulphur powder is not attracted by magnets, it is left behind. In a similar manner, an iron filling and sand mixture can be separated using a magnet.
Evaporation:
The process of evaporation is used to separate mixtures, most frequently a mixture of a solvent and a soluble material. This approach involves heating the solution until the organic solvent turns into a gas and mainly displaces the solid residue.
We cannot separate salt from water by filtration if we have a mixture of common salt and water. This is because common salt dissolves completely in water and is not insoluble in it. Evaporation allows us to recover common salt from a salt water mixture.

In a porcelain dish, a solution of common salt and water is gently heated with a burner. Water vapours will form in the salt solution and escape into the atmosphere. When all of the water in the common salt solution evaporates, the common salt remains in the porcelain dish as a white solid.
Evaporation is a large-scale process used to extract common salt from seawater. Sea water is trapped and allowed to stand in shallow lakes. The heat of the Sun gradually evaporates the water in shallow lakes, leaving common salt behind as a solid. Many salts are dissolved in sea water. When sea water evaporates, we get a salt mixture. Further purification of this salt mixture yields common salt.
Distillation:
Distillation is a simple physical separation method based on the difference in boiling temperatures of the components of a solution. The solution is heated at a selective boiling temperature that forces one of the components to enter the gaseous phase and is thus separated from the solution. This vapour-phased component is further condensed and collected separately.
By this process, non-volatile impurities and also liquids having a sufficient difference in boiling points are separated from volatile liquids.

Principle of Distillation
On heating, a liquid starts converting into its vapour phase at its boiling point. It involves heating a liquid to generate vapour and then cooling it to return it to its liquid state.
Application of distillation
- Many water purification procedures rely heavily on distillation. This approach is used by several desalination plants to get drinking water from seawater.
- Distilled water is used in lead-acid batteries and low-volume humidifiers, among other things.
- Distillation is used to make several perfumes and food flavourings from herbs and plants.
- Oil stabilisation is a sort of distillation that lowers the vapour pressure of crude oil, allowing it to be safely stored and transported.
- On a larger scale, distillation is used to purify liquid products derived through chemical synthesis.
Filtration:
Filtration is the process of removing insoluble solids from a liquid using filter paper. Filtration is a technique for removing insoluble substances from a liquid. Using a glass rod as a guide, the insoluble solid and liquid mixture is poured into a filter paper cone fixed in a funnel. The liquid flows through the filter paper and collects in the beaker beneath the funnel. The solid particles do not pass through the filter paper and are left behind. The solid and clear liquid are separated from an insoluble solid in a liquid mixture.

Filtration can also be used to separate a sand and water mixture. When a sand and water mixture is poured onto a filter paper fixed in a funnel, clear water passes through the filter paper and collects as filtrate. The sand is left behind as a residue on the filter paper.
In everyday life, various types of filters are used, such as wire mesh, cotton, muslin cloth, and tea strainers.
To separate used tea leaves, strain the prepared tea through a tea strainer. The tea strainer contains a wire mesh that serves as a filter. The liquid tea passes through the tea strainer's small holes and collects in the cup below. The tea leaves are not strained by the tea strainer. The tea leaves are left on the tea strainer. Filtration is used to separate used tea leaves from prepared tea.
Types of Filtration:
Based on the conditions in which a particular filtration process is performed, the various types of filtration are as follows:
- Gravity filtration
- Vacuum filtration
- Centrifugal filtration
- Hot filtration
- Cold filtration
- Mechanical filtration
- Conventional filtration
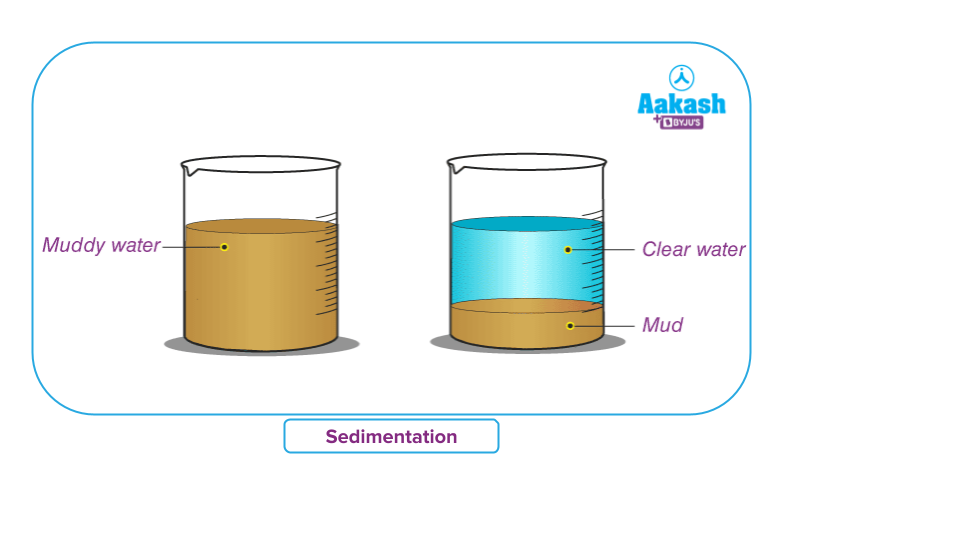
Sedimentation:
The process of sedimentation is when heavier contaminants in a liquid, usually water, fall to the bottom of the container carrying the combination. The process takes some time to finish.
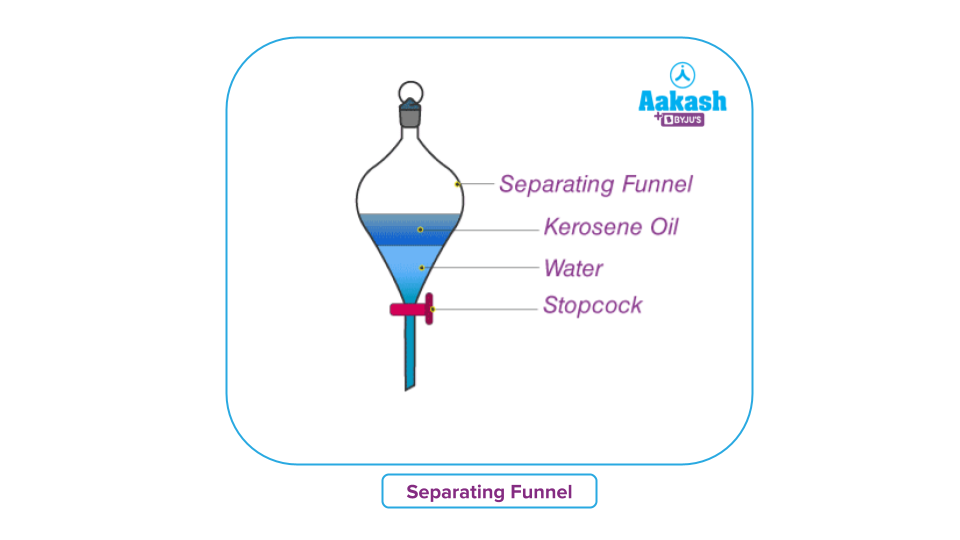
Separating Funnel:
A separating funnel is primarily used to separate two immiscible liquids. The mechanism works by exploiting the unequal density of the particles in the mixture. Using this technique, oil and water can be easily separated.
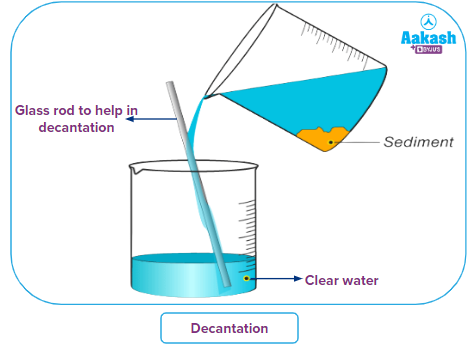
Decantation:
Decantation is the separation of liquid from solids and other immiscible (non-mixing) liquids by separating the liquid layer on top from the solid or liquid layer below.
This method can also be used to separate two non-mixing liquids, such as oil and water. When we maintain the oil and water mixture in a container, we get two distinct layers, with the water at the bottom and the lighter oil at the top. The oil layer on top can be poured into a different vessel, leaving the water layer at the bottom.
If we leave a bucket of water with sand or mud in it for a while, we'll see that the insoluble particles settle to the bottom of the bucket. The term "immiscible liquid" refers to liquids that do not mix to produce a solution.
Examples of Decantation:
The decantation process is helpful in our daily lives in many ways. Here are some examples of this process.
- To avoid losing flavour, red wine is decanted from a crystal of potassium bitartrate.
- The cream that has risen to the top of the milk is decanted. It permits cream and milk to be separated. In the cheese industry, this is used.
- To acquire a sample of water, muddy water is moved to another container.
- Decantation plays an important role in nanotechnology.
- It is used to clean liquids by removing insoluble particles from the sediment.
- It is used in the sugar industry to granulate sugarcane into sugar.
- It permits lipids to be removed from source materials during the grape vinegar production process.
- When doing diagnostic testing, it is used to separate plasma from the blood.
- The froth flotation method makes use of it. The froth flotation method is a separation procedure that separates hydrophobic and hydrophilic components from one another.
- Mercury is sometimes dumped in bodies of water, resulting in hazardous water that is unfit for human consumption. The procedure of Decantation is used to eliminate mercury.
Sublimation:
Sublimation is the process of converting a substance from a solid to a vapour state directly without passing through the liquid phase on heating and vice versa on cooling.

The process of sublimation is used for those substances which sublimate on heating from a non-volatile substance. Organic compounds that have covalent bonds are easily sublimate compared to ionic compounds.
De-sublimation, or deposition, is the inverse of this process, in which the gas transitions directly towards the solid phase. This is an endothermic phase transition that occurs when the heat and pressure of a substance fall below their triple point. As a result, energy is given away in the reversal process.
Sublimation refers to a solid substance that transforms into a gas. Sublimate is the solid that results from the cooling of vapour. It is critical to remember that the term "sublimation" only applies when a completely physical change of state occurs.
Example:

Principle of Sublimation:
Sublimation is based on the principle that solids have a weak intermolecular force, resulting in a higher vapour pressure, which changes them to a vapour state directly.
Examples of sublimation:
- Sublimation is demonstrated by dry ice, which is a frozen form of carbon dioxide. When dry ice is exposed to the air, it transforms from a solid to a gaseous state.
- Iodine treatment: The iodine crystals have been converted into the typical purple gas under laboratory heating.
- Corrosive gas sublimation: Some metallic vapours, like mercuric chloride, will inversely sublimate, which is a common degrading step in alchemical activities.
- Frost formation: Water vapour undergoes reverse sublimation at very cold temperatures, forming ice crystals on surfaces, which is known as "frost."
- The continuous snow on the mountain peaks persists in a semi-solid state, which allows it to revert to vapour without going through its liquid condition.
Crystallisation:
Crystallization is the process through which a substance's atoms or molecules arrange themselves from a solution into a well-defined three-dimensional lattice, reducing the system's overall energy.
It is used for the purification of solid substances.
Examples: Fractional (multiple) crystallization can be used to purify sugar from its mixture with common salt using ethanol as a solvent. The salt is insoluble in ethanol and so precipitated out of the solvent.
The sugar can be crystallized from the ethanol solution.
Sometimes the crystals formed are coloured due to the presence of coloured impurities. In this situation, dissolve the crystals in the same solvent and add a small amount of charcoal to them. Then boil the mixture for 15 to 20 minutes and filter away the carbon. Charcoal absorbs all the coloured impurities and gives a colourless solution containing the pure substance.

Principle of crystallization:
Crystallization is based on the principle of the difference in solubilities of compounds and impurities in a particular solvent.
Solutes usually tend to be more soluble in hot solvents than in cold solvents. If a saturated hot solution is allowed to cool without any disturbance, the decreased solubility at low temperatures forces the solute out of the solution slowly. The outgoing solute tends to grow into a pure crystal. The solid is filtered and dried. Slow cooling results in the formation of large crystals.
Application of crystallization:
- Sea water purification
-
Alum purification
-
Chiral isomers are also separated using crystallization
- Crystallization of salts is the most common use. It is also a low-budget procedure. Two utilizations of the procedure: purification of compounds and synthesis of crystals.
Chromatography:
Chromatography is an important technique extensively used to separate mixtures into their components, to purify compounds and to test the purity of compounds.
Terms used in Chromatography
- An adsorbent is a solid substance that is used to bind solute molecules from a liquid or a gas phase.
- Adsorbate: A substance that binds with an adsorbent during adsorption is an adsorbate.
- Mobile phase and stationary phase: The mobile phase is the solvent that moves through the column, while the stationary phase is the material that remains fixed inside the column.
Types of chromatography
Chromatography is classified into two categories based on the principles involved.
- Adsorption chromatography
- Partition chromatography
Adsorption chromatography
It is based on the principle that various components (adsorbates) in a mixture bind to a suitable adsorbent (silica gel, alumina) to a different extent. Some components are more strongly adsorbed than others.
Adsorbates dissolved in a solvent hence travel through the column at different rates and thus get separated with respect to time.
On the principle of differential adsorption, there are two main types of adsorption chromatography.
- Column chromatography
- Thin layer chromatography
Column chromatography
The adsorbent is filled into a cylindrical column at regular intervals. The mixture is dissolved in a suitable solvent and added slowly at the top of the column to flow under gravity. Because of the differential adsorption of substances on the adsorbent, the compounds move along the column at various rates, which allows them to be separated into fractions in column chromatography.
Thin layer chromatography
A thin-layer chromatography plate is used to separate substances in a mixture. In thin layer chromatography, the adsorbent is coated as a thin paste on an inert plate and placed in a glass chamber containing solvent at the bottom. Components of a mixture move to various distances with the solvent (eluent) due to capillary action depending on their degree of adsorption.


Partition chromatography
The components of a mixture are continuously differentially partitioned between the stationary and mobile phases in partition chromatography. It involves paper chromatography.
Paper chromatography
Partition chromatography is the principle of paper chromatography in which the components are spread or partitioned between the liquid and stationary phases. Synge and Martin first discovered paper chromatography in 1943. Paper chromatography is a chromatography technique that uses paper sheets or strips as the adsorbent and the stationary phase through which a solution is made to flow.


Types of Paper Chromatography
- Ascending Paper Chromatography: Soluble travels in an upward direction in this technique.
- Descending Paper Chromatography: In this technique, due to gravity and capillary action, the flow of solvent moves downward.
- Ascending-Descending Paper Chromatography:- After a certain point, the solvent moves in two directions in this kind of paper chromatography. The solvent moves upwards on the paper that has been folded over a rod at first, then crosses the rod and continues its downward journey.
- Radial or Circular Paper Chromatography: The sample is placed in the circular filter paper's centre. The filter paper is tied horizontally on a Petri plate containing the solvent once the spot has dried.
- Two Dimensional Paper Chromatography: Two-dimensional paper chromatography can be used to separate substances with similar rf-values.
Application of chromatography
- Water samples are tested for purity.
- Vitamins and preservatives are separated and analyzed in the food industry.
- Used in forensic science to detect a small amount of substances in the stomach.
Solvent Extraction:
The process of transferring a compound from one solvent to another due to differences in solubility or distribution coefficient between these two immiscible (or slightly soluble) solvents is known as solvent extraction.
It is a method of quantitative compound separation.
Solute from the original solvent is transferred into the extracting solvent when the extracting solvent is stirred with a solution containing solute.
When the stirring is stopped, the solvent separates into a separate layer, which now contains the solute of interest.
It provides a better separation effect than chemical precipitation and a higher degree of selectivity and faster mass transfer than the ion exchange method when compared to other separation methods.
When compared to distillation, solvent extraction has several advantages, including low energy consumption, large production capacity, quick action, easy continuous operation, and ease of automation.
Commonly used solvents
- Diethyl ether
- Chloroform
- Dichloromethane
- Ethyl acetate
Uses of Solvent Extraction
- The extraction of solvents is used in the production of perfumes, vegetable oils, and biodiesel.
- It is also used to recover plutonium from irradiated nuclear fuel, a procedure known as nuclear reprocessing.
- The plutonium recovered can then be used as nuclear fuel.
Practice Problems:
Q. 1. Which substance will separate first from a mixture of sand, salt, and benzoic acid?
A. Sand
B. Benzoic acid
C. Salt
D. Sand and benzoic acid
Answer: B
Solution: Benzoic acid will be separated first by sublimation, and the other two be separated by filtration after dissolving in water.
Q. 2. Which of the following is a crystallization process example?
A. Alum purification
B. Seawater purification
C. Gas separation from air
D. None of the above
Answer: A
Solution: The crystallization procedure purifies an impure sample of alum or copper sulfate. Distillation is used to purify seawater.
Q. 3. Which of the following process is used to separate a solid which is dissolved in liquid?
A. Sedimentation
B. Filtration
C. Evaporation
D. decantation
Answer: C
Solution: Evaporation is the process that is used to separate a dissolved solid from a liquid.
Example: salt from seawater.
Q. 4. Which factors affect distillation?
A. Relative volatility
B. Surface area
C. Solubility
D. All of these
Answer: D
Solution: There are various factors that affect the distillation process, which are solubility, relative volatility, and activity coefficient, and it also depends on the surface area.
Q. 5. Chromatography is based on which principle?
A. The rate of movement of components in a column is different
B. The rate of movement of components in the mixture is different
C. The capture of one solute on the adsorbent allows it to be separated from other components
D. None of the above
Answer: B
Solution: The interaction of solute molecules with active sites on the stationary phase is the basis of chromatography. The polarity of the solutes determines this attachment or interaction. This technique demonstrates the axiom "polar like polar." Because the stationary phase is more polar than the mobile phase, the mixture's high polar compounds will be strongly attached to the stationary phase, while less polar compounds will be loosely bound. The mobile phase will elute less tightly bound compounds earlier than tightly bound compounds.
Frequently Asked Questions-FAQs:
Q. 1. Why are temperature and pressure conditions required for sublimation?
Answer: In the sublimation process, the phase transition is taking place which depends on the condition of temperature and pressure. When pressure and temperature conditions are below triple point, a solid substance directly sublimes into vapour.
Q. 2. Why is heating done in the process of crystallization?
Answer: Heating is done to form a supersaturated solution, which on cooling back will throw out substances that will combine and grow into a big crystal.
Q. 3. Which distillation techniques are used for the molecules having very little difference in boiling points?
Answer: Fractional distillation is used to separate those liquids which have fewer differences in boiling points.
Q. 4. Why is the chromatography technique preferred over other purification techniques?
Answer: It detects even a very small amount of component is present in a mixture and also detects any number of components present in a mixture.
Q. 5. How would you separate a sand and camphor mixture?
Answer: By sublimation sand and camphor mixture is separated as camphor is a volatile substance that sublimes to vapours and sand remains.


