-
Call Now
1800-102-2727
Soaps and Detergents-Definition, Properties, Types, Difference between Soap and Detergent, Practice problems, FAQs
You must have heard your teacher say, "Wash your hands frequently with soap and water for at least 20 seconds." Some of you may be wondering how these foams in soap form when we rub our hands together. Soaps have now become an indispensable part of our daily lives. The germs on our hands are eradicated when we wash them with soap. This helps us stay healthy by keeping hazardous bacteria out of our bodies.
Have you ever witnessed your mother washing your soiled clothes?
She generally adds additional supplements to the water when washing the filthy garments, and then the froth/lather forms.
Have you ever mopped your floor before? Cleaning your floors is just as important as cleaning your clothes. It is a daily task that must be completed as part of our daily schedule. A good detergent is necessary for good health. When you touch various surfaces, germs and other harmful elements come into contact with your clothing. Detergent is used to remove the impurities.
There are a variety of uses and functions which we must know about soaps and detergents.
Let’s study this in detail.


Table of content:
- What is soap?
- What is a detergent?
- Properties of soap
- Properties of detergent
- Soap-making process
- Preparation of detergents
- Types of soaps
- Types of detergent
- Cleansing action of soap and detergent
- Difference between soap and detergent
- Practice problems
- Frequently asked questions-FAQs
What is soap?
- Soap is a fatty acid salt that can be found in nature.
- Soaps are generally utilized as surfactants in washing, bathing, and cleaning, but they're also used in lubricants and textile spinning.
- Soaps are water-soluble fatty acid sodium or potassium salts. Basically, soaps are merely long-chain fatty acid salts of potassium or sodium.
- Although soap is required for proper hand washing and hygiene, the World Health Organization recommends that clean ash or sand/soil be used in an emergency.

What is a detergent?
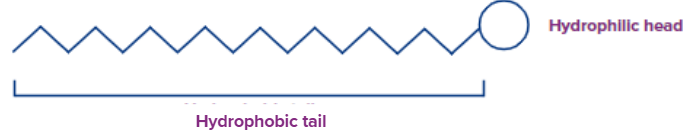
- Amphipathic substances having charged hydrophilic or polar chemicals at the extremities of long lipophilic hydrocarbon groups are known as detergents. The tail is a long lipophilic hydrocarbon group, whereas the head is a charged hydrophilic group.
- Detergents, also known as surfactants, have the ability to reduce the surface tension of water. A detergent is a potent cleaning agent because it contains one or more surfactants. Due to their chemical composition, surfactants in detergents can be designed to work well in a number of circumstances. Surfactants that are less sensitive to hardness minerals in water than soap, for example, are less prone to form a film.

Properties of soap:
- Cleaning: The most common reason people use soap is to clean themselves. A soap molecule is made up of a carbon chain that attracts oil on one end and water on the other. As a result, the cleansing ingredient in the soap should be balanced, with neither too much nor too little added during the production process.
- Emollients are chemical substances that are found in soap conditioners. The amount of soap left on your skin after you've washed and rinsed your hands is determined by the type of soap you use.
- Harder soap comes in the form of a solid bar that lasts longer.
- When soap reacts with mineral salts, soap film forms an insoluble precipitate.
- A significant component is an aroma. Aromas evoke a unique mix of personal recollections while also enriching our daily lives. Fragrances energize, relax, and, most importantly, conceal our odours.
Properties of Detergents:
- The cloud point is the temperature at which a detergent solution splits into two phases when it reaches or exceeds the critical micelle concentration.
- Critical micelle concentration(CMC) refers to the concentration where the micelles begin to form.
- Micelle molecular weight represents relative micelle size.
- The proportion of monomers in a micelle is known as the aggregation number.
- Even with hard water, detergents are effective cleaners.
Soap-making process:
- The procedure of making soap hasn't changed much over the years.
- In ancient Roman tradition, rainwater, potash, and animal tallow were mixed together. Making soap took a long time and a lot of labour. First, the fat had to be rendered (melted and filtered).
- After that, a potash solution was applied. Because water and oil do not mix, this mixture had to be constantly swirled and heated in order to keep the fat melted. Saponification, a chemical reaction, took place gradually. In this procedure, the triglyceride fats are hydrolyzed into free fatty acids, which then react with the alkali to produce crude oil.
- Soap is a free mixture of soap salts, excess fat or alkali, water, and glycerol (glycerin). Glycerin is a useful by-product that can be left in the soap as a finishing touch or removed and used for other reasons like softening.
Let us study this saponification reaction in brief-
Saponification reaction:

Preparation of detergents:
By reacting dodecyl alcohol (dodecanol) with sulfuric acid, a sodium alkyl sulphate known as sodium dodecylsulfate will be formed.
A reaction with sodium hydroxide converts the resultant dodecylsulfate to the sodium salt.
Types of soap:
Soaps are made almost entirely by heating fats or oils with soluble hydroxide. To generate variances, different basic materials are employed.
- Toilet soaps: These are manufactured with higher-quality fats and oils, and particular attention is paid to removing excess alkali. Colour and smells are added to make these more appealing.
- Translucent soaps: These are made by smashing microscopic air bubbles before they harden to make floating or transparent soaps. Dissolve the soap in ethanol and then evaporate the surplus solvent to make translucent soap.
- Medicated soaps: Medicated soaps are made up of substances that have medicinal properties. Certain soaps contain deodorants to keep you feeling fresh and clean.
- Shaving soaps: Glycerol is used in shaving soaps to keep them from drying out too quickly. Rosin is used as gum in their production.
- Laundry soaps: Fillers in laundry soaps include sodium rosinate, sodium silicate, borax, and sodium carbonate.
Types of detergents:
Synthetic detergents are generally divided into three groups:
Anionic detergents, cationic detergents, and non-ionic detergents are the three types of detergents.
- Anionic Detergents: The sodium salts of anionic detergents are long-chain alcohols or hydrocarbons that are sulphonated. By neutralizing the alkyl hydrogensulphate produced by processing long-chain alcohols with strong sulfuric acid, anionic detergents are created. Similar to how alkyl benzene sulphonic acids are neutralized, alkyl benzene sulphonates are produced by neutralizing alkyl benzene sulphonates with alkali.

- The anionic component of the molecule mediates the cleansing action of anionic detergents. Alkylbenzenesulphonates sodium salts are a typical anionic detergent. The home is where they are most frequently used. In toothpaste, anionic detergents are also present.

- Cationic Detergents: The quaternary ammonium salts of amines with anions like acetates, chlorides, or bromides are known as cationic detergents. Long hydrocarbon chains and a positively charged nitrogen atom identify the cationic component. This technique leads to cationic detergents. The cationic detergent cetyltrimethylammonium bromide is frequently found in hair conditioners.


- Non-ionic Detergents: There are no ions in the formula of non-ionic detergents. When stearic acid and polyethene glycol mix, a detergent is formed.
![]()
There are non-ionic liquid dishwashing detergents on the market. This sort of detergent works in a similar way as soaps in terms of cleaning. Grease and oil are also removed through micelle formation.

The cleansing action of soap and detergents:
Think of the situation with soap solutions, A higher fatty acid that may be written as RCOO-Na+ is what makes up soap. One frequent component in bar soaps is sodium stearate, which is written as CH3(CH2)16COO-Na+. When dissolved in water, it splits into RCOO- and Na+ ions. The RCOO- ions, on the other hand, are composed of two components: a hydrophilic polar group RCOO- (also known as a polar-ionic "head") and a lengthy hydrophobic (water-repelling) hydrocarbon chain R. (water-loving).
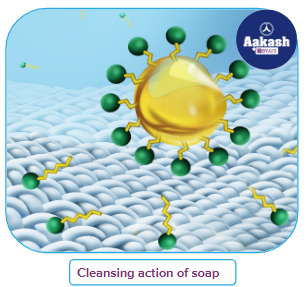
A central core that resembles a hydrophobic hydrocarbon makes up a micelle. With the hydrophobic portion of the stearate ions in the oil droplet and the hydrophilic portion of the stearate ions spreading out of the grease droplet like bristles, soap molecules form micelles surrounding the oil droplet to provide cleaning action. Because the polar groups may interact with water, the oil droplet surrounded by stearate ions is now sucked in water and released from the filthy surface. The globules' negatively charged surface prevents them from sticking together and forming aggregates.
Detergents are surface-active agents or surfactants with a hydrophobic "tail" and a hydrophilic "head," and they operate according to a similar mechanism. To keep their tails together and out of the solution phase, surfactant molecules in aqueous solutions often take the form of "micelle" structures. The majority of dirt's oily molecules can enter the centre of these micelles, thereby dispersing them in water and allowing them to be washed away. This process works much more quickly with some mechanical assistance, which is why scrubbing, mixing, and other operations are commonly required.
Difference between soap and detergent:
|
Soap |
Detergent |
|
A "-COONa" group is joined to a fatty acid with a lengthy alkyl chain. |
A “-SO3Na” group is coupled to a long alkyl chain. |
|
They are inefficient in saline and hard water. |
They continue to work well in hard and salty water. |
|
Soaps are biodegradable in nature |
The hydrocarbon chain in non-biodegradable detergents is branching. |
|
They have a predisposition for developing scum in hard water environments. |
These compounds have no effect on the formation of scum |
|
They are produced using natural resources like vegetable oils and animal fats. |
Detergents made from synthetic compounds are utilised. |
|
Soap is a product that benefits the environment because it degrades naturally. |
A thick foam that is produced by these compounds can harm aquatic life. |
|
Examples of soaps include sodium palmitate and sodium stearate. |
Detergents like sodium lauryl sulphate and deoxycholic acid are two examples. |
Practice problems:
Q1.Critical micelle concentration is_____________________.
A. the concentration below which micelle formation takes place.
B. the concentration above which micelle formation takes place.
C. the concentration at which micelle formation takes place.
D. the concentration at which the surface became unsaturated.
Answer: B
Solution: Critical micelle concentration is defined as the concentration above which micelle formation takes place. The surface becomes saturated at the CMC point, and adding more surfactant molecules has no effect on the surface tension.
Q2. Soft soaps are restricted to hot processes because of their _____________.
A. Low alkaline nature
B. High alkaline nature
C. High solubility in oil
D. High solubility in water
Answer: D
Solution: Because of their higher water solubility, soft soaps are restricted to hot process constraints. Generally, soaps made from potassium hydroxide are made from cold procedures. The soft soaps which we generally use are not soluble in hard water.
Q3. ‘Builder’ in detergent works _______.
A. to improve surfactant washing efficiency
B. as an ion exchanger for calcium ions
C. as detergent additives that do not have the potential to improve the wash
D. to keep dirt from re-settling on the garments
Answer: A
Solution: Builder (former) works to improve the surfactant's washing efficiency in order to disable the minerals that cause water hardness. This material is added to the wash water to remove calcium and magnesium ions (hardness).
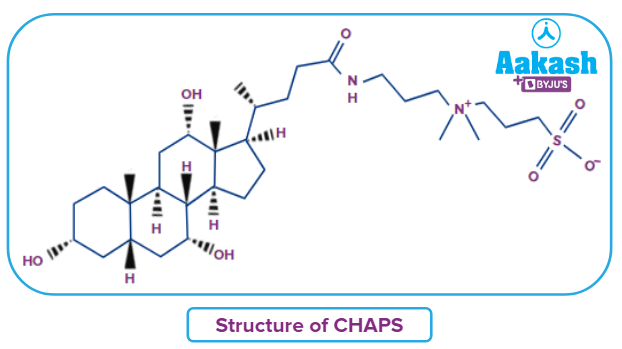
Q4. _____________is a steroid-based zwitter ionic detergent.
A. Sodium dodecyl sulfate
B. CHAPS [(3-((3-cholamidopropyl) dimethylammonio)-1-propanesulfonate) ]
C. cetyltrimethylammonium bromide
D. sulfobetaine
Answer: B
Solution: Zwitter ionic detergents have both ionic and nonionic properties.CHAPS is an example of a steroid-based zwitter ionic detergent. Below is the structure of CHAPS.
Frequently asked questions-FAQs
Q1. Who invented the versatile substance “soap”?
Solution: In 2800 B.C., the Babylonians were the first to invent soap. They discovered that combining fats, specifically animal fats, with wood ash generated a cleaner product. The first soap was used to wash textile industry wool.
Q2. Is soap considered a detergent?
Solution: The components are the main distinction between soap and detergent. Biodegradable components such as oils and fats are used to make soaps. Surfactants, optical brighteners, and fragrances are among the synthetic compounds used in detergents.
Q3. Is bleach considered a detergent?
Solution: Bleach and detergents are two different things. Your clothes will be clean if you use bleach and detergent together. Although detergent can be used alone, bleach improves cleaning performance and is generally used for removing tough stains over white clothes.
Q4. What do you mean by laundry detergent made from plants?
Solution: Because they use natural components produced from plants, plant-based detergents are referred to as green or eco detergents and thus considered laundry detergents made from plants.


