-
Call Now
1800-102-2727
Enzymes: Catalyst, Characteristics, and Structure of Enzymes, Inorganic Catalyst vs Enzymes, Examples, Types, and Classification of Enzymes, Practice Problems, and FAQs
Have you heard of people who have a difficult time consuming dairy products like milk, cheese, curd, etc? Do you know the reason behind this? Well, these people have lactose intolerance. Lactose is the main carbohydrate in milk products and the lactase enzyme in our digestive system digests it. But people having lactose intolerance do not produce lactase enzymes and they have difficulty consuming milk products.

Fig: Lactose intolerance
Likewise, there are several enzymes in the human body and all have their specific functions. Most of the enzymes are proteins which means that they are composed of polypeptide chains. Different enzymes have different structures and functions and their substrate molecules are also different.
In this article we will discuss different types of enzymes, their structure, and their function.
Table of contents
- Catalyst
- Enzyme
- Characteristics of enzymes
- Structure of enzyme
- Inorganic catalyst vs Enzymes
- Examples of enzymes
- Types of enzymes
- Classification of enzymes
- Practice Problems
- FAQs
Catalyst
Catalysts are substances that accelerate the production of a product during a reaction. In contrast to reactants, which take part in the reaction and are utilised in it, catalysts take part in the process but are not used in it. Catalysts are of two types:
- Inorganic catalyst
- Organic catalyst
Inorganic catalysts
Inorganic catalysts have metal ions in them, such as platinum and rhodium. Catalysts can also be made from semi-metallic substances including silicon, aluminium, and boron.
Organic catalysts
Organic catalysts are enzymes that are also known as catalytic proteins. Therefore, the proteins that act as catalysing agents in living systems are called catalytic proteins. They enhance the speed of the chemical reactions that occur inside the living body. The organic catalysts or enzymes are present in all living organisms. Examples include pepsin and trypsin which are responsible for the hydrolysis of proteins in the digestive system of humans.
Enzyme
Enzymes are described as the biological polymers that catalyse biochemical reactions. Most enzymes are proteins having catalytic properties that are essential to carry out various biochemical reactions in the body. A number of different enzymes are essential for maintaining life and carrying out metabolic processes and other chemical reactions within the cell.
During an enzymatic reaction, enzymes react with the substrate and convert these substrate molecules into other distinct molecules, which are known as products.
All enzymes, except ribozymes, are composed of proteins. The ribozymes are ribonucleic acid (RNA) molecules which have enzymatic activities.
Enzymes are present in all types of tissues and fluids of the body. Intracellular enzymes are responsible for all catalysis occurring in metabolic processes within the cell. On the other hand, extracellular enzymes function outside the cell. They are synthesised by the cell and secreted to the outer environment of a cell. For example, the enzymes present in the plasma membrane regulate the catalytic reactions occurring in a cell, whereas the enzymes of the circulatory system regulate the process of blood coagulation.
Characteristics of enzymes
- Enzymes are usually proteins in nature and act as catalysts. Exceptions include Ribozymes (RNA acting as enzymes)
- Substrates are the reactants of the reaction which interact with the enzyme and get converted into products.
- Enzymes have a high degree of specificity for their substrates, i.e, a particular enzyme can bind to a particular substrate only.
- The catalytic power of enzymes is very high and due to this, they catalyse a reaction even in the presence of small amounts.
- They are known to enhance the speed of a chemical reaction despite remaining unchanged during the whole reaction.
- The factors that affect the efficiency and action of enzymes included temperature, pH, and inhibitors.

GIF: Enzyme reaction
Structure of enzyme
As the majority of enzymes are protein in nature, they can exist in the following structures:
- Primary structure
- Secondary structure
- Tertiary structure
- Quaternary structure
Primary structure
It is an amino acid sequence of the protein that is joined together by peptide bonds. Enzymes are not functionally active in their primary structures.
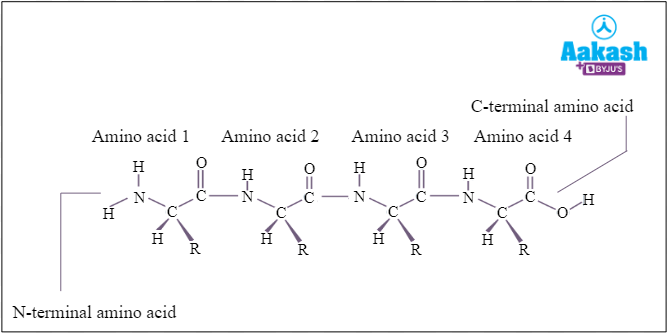
Fig: Primary structure of proteins (Enzymes)
Secondary structure
The polypeptide chain folds upon itself to form an alpha helix or a beta-pleated structure, stabilised by hydrogen bonds. In secondary structure, enzymes are not functionally active because of the absence of pocket-like foldings.
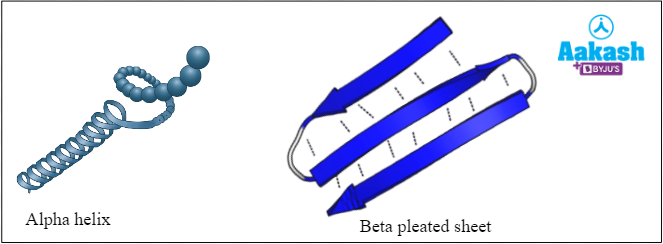
Fig: Secondary structure of proteins (Enzymes)
Tertiary structure
It is a polypeptide with a secondary structure that further folds upon itself to form a compact 3D structure. Crevices or pockets are made during this folding which can serve as active sites for the binding of the substrate. Therefore, enzymes become fully functional in tertiary structure.

Fig: Tertiary structure of proteins (Enzymes)
Quaternary structure
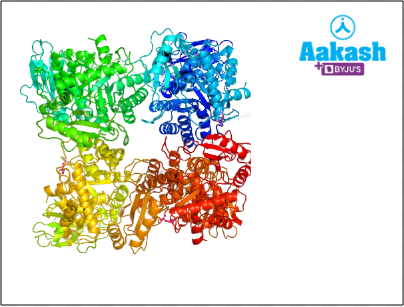
Some enzymes exist as a quaternary structure whch is composed of units of tertiary structure that aggregate to form homo or hetero multimers. It is the highest level of structure that is composed of more than one polypeptide chain. DNA polymerase is an enzyme that exists as a quaternary structure and consists of four subunits (Pol2, Dpb2, Dpb3, and Dpb4).
Active site
An active site of an enzyme is a crevice or pocket into which the substrate fits. For example, the enzyme DNA polymerase has a “Polymerase active site’.
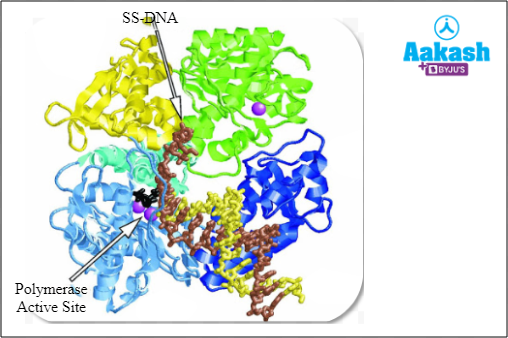
Fig: DNA Polymerase
The active site is the binding site of the substrate. The active site of the enzyme allows the substrate to fit into the enzyme like a key fits into the lock. So, enzymes are highly specific. The lock is the active site and the substrate is the key. This is the binding site of the substrate. If a substrate that is not specific for an enzyme tries to bind the active site, it will not fit in. Only the specific substrate of an enzyme can fit in the active site of that particular enzyme. Hence, enzymes are highly specific.

GIF: Enzyme-substrate reaction
Every enzyme has an active site to which the substrate binds and results in the formation of a short-lived highly reactive enzyme-substrate complex. After this complex formation, there is the formation of enzyme-product complex (EP). In the end, the enzyme-product complex dissociates into the product (P) and the enzyme released remains unchanged to be used for the next substrate.

GIF: Enzyme-substrate complex
The active site is formed by few amino acid residues and their side groups which are brought closer due to folding of the polypeptide chain. These amino acid residues, when brought together, are most efficient in catalysing a particular enzymatic reaction. For example, the active site of aldolase consists of glycine, histidine and alanine and that of pyruvic oxidase consists of aspartic acid, cysteine and alanine.
Inorganic catalyst vs Enzymes
|
Inorganic catalyst |
Enzymes |
|
Inorganic catalysts are metal ions that speed up the rate of a chemical reaction. |
Enzymes are proteins that catalyse the biochemical reaction. |
|
They work efficiently at high temperatures. |
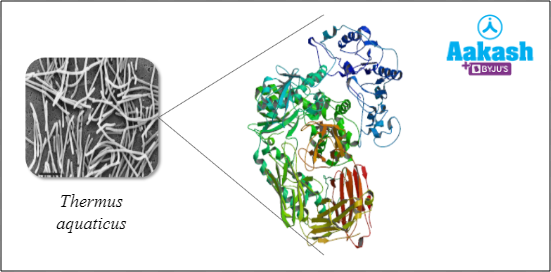
They get damaged at high temperatures Exceptions include enzymes in the organisms found in hot vents and sulphur springs. They are stable up to 80°–90°C. For example, Taq polymerase isolated from Thermus aquaticus (bacteria residing in hot springs)
Fig: Taq polymerase |
|
They work efficiently at high pressure. Examples include platinum in the catalytic converter.
Fig: Platinum used in catalytic converter |
They get damaged at high pressures. Examples include Chymosin, trypsin, lipases, etc. |
|
Have low molecular weight. |
Have very high molecular weight. |
|
A single catalyst can catalyse many types of chemical reactions. |
A particular enzyme can catalyse only a specific type of biochemical reaction. |
|
Not affected by poisons or radiations. |
Inhibited or inactivated by poisons and radiations. |
|
Cannot be regulated by regulatory substances. |
Can be regulated by regulatory substances. |
Examples of enzymes
Some of the examples of enzymes are given below:
|
Enzyme: RuBisCO Most abundant enzyme found in the biosphere which is present in the chloroplast of plant cells and is mainly responsible for photosynthesis in plants. Found in: Plants Function: Carbon fixation |
Fig: RuBisCo |
|
Enzyme: Amylase Found in: Wheat Function: Starch digestion |
Fig: Amylase in wheat helps in baking |
|
Enzyme: Chymosin or rennin Found in: Rennet (set of enzymes found in the stomach of ruminants), stomach of human infants Function: Coagulation of milk |
Fig: Chymosin in rennet is used for cheese making |
|
Enzyme: Alcohol dehydrogenase Found in: Yeast cells Function: Alcoholic fermentation of pyruvic acid to form ethanol |
Fig: Alcohol dehydrogenase in yeast helps in production of alcoholic beverages |
|
Enzyme: Lipases Found in: Intestinal juice of humans, laundry detergent Function: Break down fats into fatty acids and glycerol |
Fig: Lipase in laundry detergent |
|
Enzyme: Microbial peroxidases Location: Microbes Function: Break down toxic peroxides to harmless products |
Fig: Microbial peroxidases are used for waste water treatment |
Types of enzymes
Around 2000 enzymes are known. There are two types of enzymes
- Simple enzymes
- Conjugated enzymes
Simple enzymes
These enzymes are composed of only amino acids. Examples include pepsin which is a digestive enzyme. This enzyme is entirely made up of amino acids and aids in protein digestion.

Fig: Pepsin enzyme
Conjugated enzymes
Conjugated enzymes are composed of amino acids and a non-protein group. This non-protein group is also known as a prosthetic group. After removing the prosthetic group from the conjugated protein, it leaves behind the protein component only which is also known as apoenzyme. It is biologically inactive.
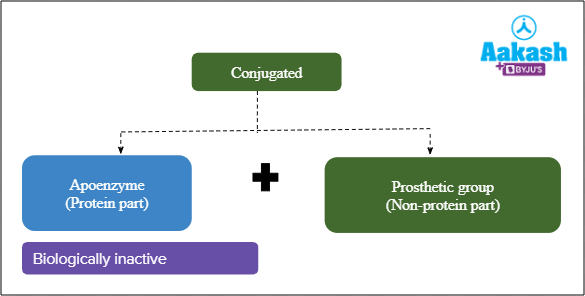
Fig: Conjugated enzyme
The apoenzyme and the prosthetic group together form the biologically active holoenzyme.
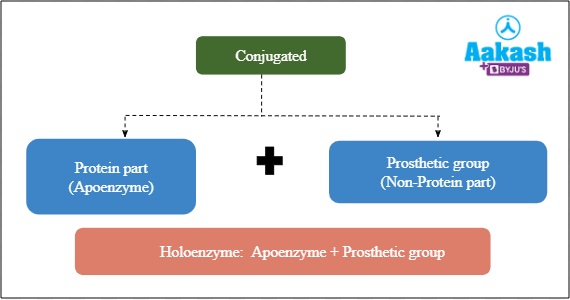
Fig: Holoenzyme
Prosthetic group
They are the non-protein part of a conjugated enzyme that makes it catalytically active. These are organic or inorganic compounds that are bound to the apoenzyme and are a part of the active site of an enzyme. When the prosthetic group is eliminated from the conjugated enzyme, the catalytic activity of an enzyme is lost. Prosthetic groups can be of the following types:
- Cofactors
- Coenzymes
Cofactors
These are inorganic compounds that are required for enzyme activity. They form coordination bonds with the side chains at the active site and with the substrate. A coordination bond is a type of covalent bond that is formed when metal ions link with organic compounds. In this type of bond, the pair of electrons is used from the same atom. For example zinc serves as a cofactor for the enzyme carbonic anhydrase.
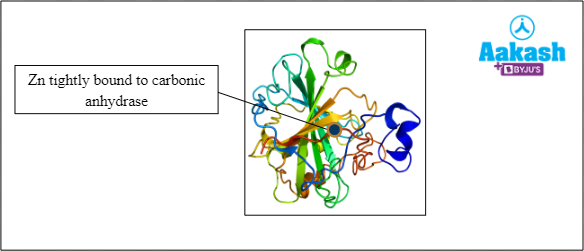
Fig: Carbonic anhydrase
Haem is an iron containing cofactor of enzymes such as peroxidase, catalase, cytochromes, haemoglobin, etc.
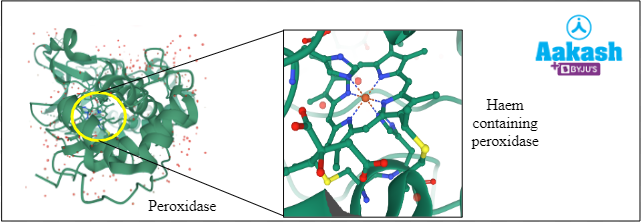
Fig: Peroxidase enzyme
Coenzymes
These are organic compounds that are transiently or temporarily bound to the apoenzymes. Coenzymes are mostly compounds of the vitamin B complex. Examples include coenzymes NAD and NADP which have niacin as an essential component.

Fig: Coenzyme
Classification of enzymes
Naming the enzymes is called nomenclature of enzymes. The enzymes usually have ‘-ase’ as suffixes such as lactase, dehydrogenase, anhydrase, etc. Although there are exceptions such as trypsin where common names are used. Common names provide little information about the reactions that they catalyse. An International Commission on Enzymes was established to create a systematic basis for enzyme nomenclature to avoid confusion at a local level. Based on the type of chemical reaction catalysed, enzymes are classified into six classes:
- Oxidoreductases
- Transferases
- Hydrolases
- Lyases
- Isomerases
- Ligases
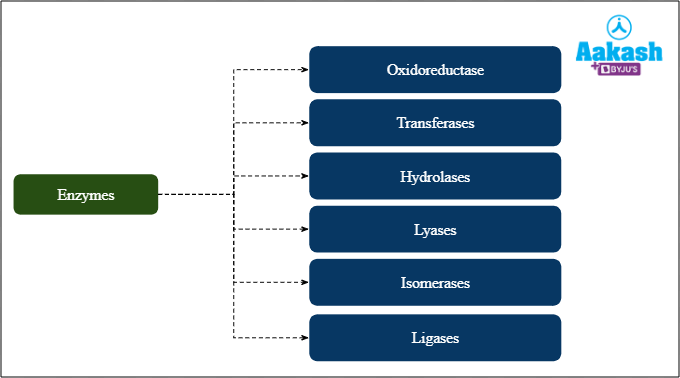
Fig: Classification of enzymes
Oxidoreductases
These enzymes catalyse oxidation/reduction of their substrate and act by adding or removing H+ ions to or from the substrate. Examples include oxidases, dehydrogenases, oxygenases, and peroxidases.

Fig: Action of oxidoreductases
Transferases
These enzymes catalyse reactions that involve the transfer of groups (other than hydrogen) from one molecule to another. Examples of such groups include amino, carboxyl, carbonyl, methyl, phosphoryl, and acyl (RC=O). They have a common prefix: Trans. Examples include transcarboxylase, transaminases, kinases, and phosphorylases.
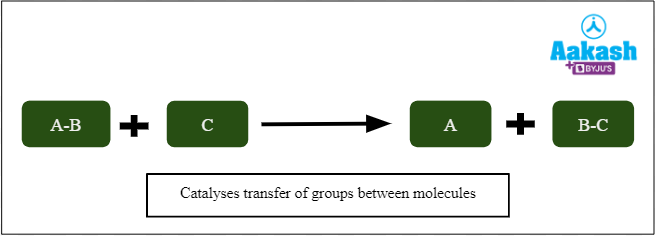
Fig: Action of transferases
Hydrolases
They catalyse the reactions in which the cleavage of bonds is accomplished by adding water. Examples include phosphodiesterases, phosphatases, and peptidases.
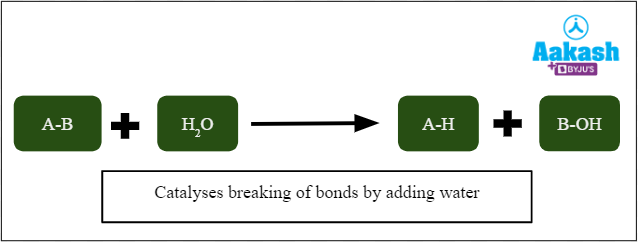
Fig: Action of hydrolases
Lyases
These enzymes catalyse the breaking of C-C, C-O, C-N, C-S, and other bonds by means other than hydrolysis or oxidation. These bonds are cleaved by the process of elimination and result in the formation of a double bond or a new ring, or adding groups to double bonds. Examples include aldolases, synthases, decarboxylases, etc.

Fig: Action of lyases
Isomerases
Isomerases catalyse several types of intramolecular arrangements and yield isomeric forms. Examples include mutases, cis-trans isomerase, epimerases, and racemases.
Fig: Action of isomerases
Ligases
Ligases catalyse the formation of C-C, C-S, C-O, and C-N bonds. The energy for these reactions is always supplied by ATP hydrolysis. Another common name is synthetases as they are always used to synthesise new molecules.

Fig: Action of ligases
Practice Problems
- Identify the correct statement from the following options.
- Holoenzyme = Apoenzyme + Coenzyme
- Coenzyme = Apoenzyme + Holoenzyme
- Holoenzyme = Coenzyme + Cofactor
- Apoenzyme = Holoenzyme + Coenzyme
Solution: Holoenzyme is the functionally active form of a conjugated enzyme which is composed of two parts. It is made up of a non-protein component called the prosthetic group and a protein component called the apoenzyme. Prosthetic groups can be inorganic compounds known as cofactors or organic compounds known as coenzymes. Therefore, coenzymes and apoenzymes combine to generate holoenzymes. Hence, the correct option is a.
2. Determine the correct statement with respect to an active site of an enzyme.
- The active site is formed by the foldings and crevices in the tertiary structure
- The substrate binds to the active site
- The enzyme catalyses a reaction through its active site
- All the above
Solution: The tertiary structure of a protein is formed when the polypeptide chain folds itself and produces several crevices and pockets. One such crevice or pocket where the substrate can fit and attach to an enzyme is called the active site. The binding of substrate to the active site activates the enzyme and this catalyses the reaction. Therefore, all the given statements are true. Hence, the correct option is d.
3. Which of the following reactions is catalysed by lyases?
- formation of C-C, C-S, C-O, and C-N bonds
- transfer of groups (other than hydrogen) from one molecule to another
- breaking of bonds by adding water
- breaking of C-C, C-O, C-N, C-S, and other bonds by means other than hydrolysis or oxidation
Solution: Lyases catalyse the breaking of C-C, C-O, C-N, C-S, and other bonds by means other than hydrolysis or oxidation. These bonds are cleaved by the process of elimination and result in the formation of a double bond or a new ring, or adding groups to double bonds. Examples include aldolases, synthases, decarboxylases, etc. Hence, the correct option is d.
4. Kinases are examples of which of the following types of enzymes?
- Ligases
- Transferases
- Lyases
- Isomerases
Solution: Kinases are enzymes that transfer the transfer of a phosphate group from a high energy compound to a substrate. Thus, it is a type of transferase enzyme. These enzymes catalyse reactions that involve the transfer of groups (other than hydrogen) from one molecule to another. Examples of such groups include amino, carboxyl, carbonyl, methyl, phosphoryl, and acyl (RC=O). They have a common prefix: Trans. Examples include transcarboxylase, transaminases, kinases, and phosphorylases. Hence, the correct option is b.
FAQs
- What are pancreatic enzyme supplements?
Answer: Pancreatic enzyme supplements are tablets or capsules having a combination of pancreatic lipases, amylases and proteases. These are administered to people in whom the pancreas is unable to secrete enough digestive enzymes and hence supplements need to be given. The capsules consist of multiple beads, each of which are composed of the enzymes and have an enteric coating which easily dissolves in the small intestine and allows the enzymes to act on partially digested food in the small intestine.
- How many enzymes are present in the human cell?
Answer: Enzymes are protein catalysts that speed up biochemical reactions in the body. In the human cells, approximately 1300 enzymes are present.
- Which enzyme is known as the slowest enzyme?
Answer: Lysozyme is the slowest enzyme in the human body. This is because the turnover number of this enzyme is too low and that is 0.5.
- Who discovered enzymes?
Answer: Enzyme was discovered by the chemist Anselme Payen in 1833.
YOUTUBE VIDEO