-
Call Now
1800-102-2727
Carboxylic Acids - Physical Properties, Biological Significance, Uses, Practice Problems and FAQ
Hyaluronic acid, a highly hyped ingredient, has become extremely popular among new-age beauty enthusiasts and customers. Everyone is talking about its benefits, from celebrities sharing their skincare secrets to beauty influencers attempting to sell a product to their followers. And, whether knowingly or unknowingly, a product containing HA is gaining all the attention and becoming an instant hit! The craze is far too real to dismiss! Every brand has launched new products touting hyaluronic acid as one of the main ingredients, and it has thus become the most valuable player in skincare.

What does it do by the way?
Well, it is a disaccharide polymer, which comprises D-glucuronic acid and N-acetyl-D-glucosamine, linked via alternating β-(1→4) and β-(1→3) glycosidic bonds. Hence, it has the root functional group of -COOH and is a carboxylic acid. It has huge hydrating properties. In fact, it is a humectant capable of absorbing moisture from the atmosphere and retain it in the upper layer of skin, thereby making it look plump and youthful! Well, I guess that is quite enough to bring you some interest in understanding the uses of carboxylic acids and how diversified their applications can be.
Let’s get started!
TABLE OF CONTENTS
- Carboxylic Acids - Introduction
- Carboxylic Acids - Physical Properties
- Carboxylic Acids - Biological Importance
- Carboxylic Acids - Significant Applications
- Carboxylic Acids - Uses in Various sectors
- Practice Problems
- Frequently Asked Questions - FAQ
Carboxylic Acids - Introduction
Carboxylic acid is a family of chemical compounds distinguished by the existence of a (-COOH) carboxyl group. Carboxylates are carboxylic acid salts and esters. Most of the carboxylic acids of biological origin are found as esters of glycerol, that is, as triacylglycerols. Some higher members of aliphatic carboxylic acids (C12-C18) are known as fatty acids, and they are found in natural fats as esters of glycerol.
Carboxylic Acids - Physical Properties
To understand the functioning and applications of carboxylic acid, it is important to learn about its physical properties to decode its behaviour.
- Carboxylic acid molecules are polar because they include two electronegative oxygen atoms.
- They also participate in hydrogen bonding because of the presence of the carbonyl group (C=O) and the hydroxyl group.
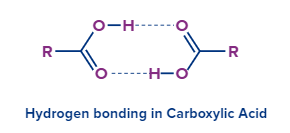
- When these compounds are placed in non-polar liquids, the hydroxyl groups of one carboxylic acid and the carbonyl group of the other form dimers due to hydrogen bonding.
- The size of carboxyl functional group-containing substances determines their solubility in water. The smaller the chemical, the higher the solubility (the shorter the R group).
- Due to their inclination to "self-associate," carboxylic acids frequently reside as dimers in non-polar fluids. Smaller carboxylic acids (1 to 5 carbons) are water-soluble, however, larger carboxylic acids have restricted solubility due to the alkyl chain's increasing hydrophobicity. In less polar solvents like ethers and alcohols, these longer chain acids are more soluble.
- Carboxylic acids have a higher boiling point than aldehydes, alcohols, and ketones with the same molecular mass.
- The higher boiling point is at times attributed to the hydrogen bonding present in the carboxylic acids. Because of their larger surface areas and proclivity for forming stable dimers through hydrogen bonding, carboxylic acids have higher boiling temperatures than water. Either the dimer bonds must be broken or the complete dimer arrangement must be vapourised for boiling to occur, considerably raising the enthalpy of vapourisation required.
- Carboxylic acids can also be dissolved in less polar organic solvents such as ether, alcohol, benzene, chloroform, and others.
- Because these substances (RCOOH) have the ability to give protons (H+), they are known as Bronsted-Lowry acids.
- The carboxylate ion (-COO-) is resonance stabilised.
- They generally have a strong sour odour. Esters of carboxylic acid have a pleasant odour and are used in perfumery.
Carboxylic Acids - Biological Significance
Carboxylic acids have a significant role in biological systems too.
- Higher carboxylic acids, called fatty acids, are beneficial to human health. Omega-6 and omega-3 are necessary fatty acids that the body does not generate. They aid in the maintenance of the cell membrane as well as the regulation of nutrient usage and metabolism.
- When we eat a meal high in unsaturated fat, glucose and other nutrients are absorbed directly into the bloodstream. If you eat a lot of saturated fat, your digestion will slow down, giving your body more time to absorb the energy and nutrients from your meal.
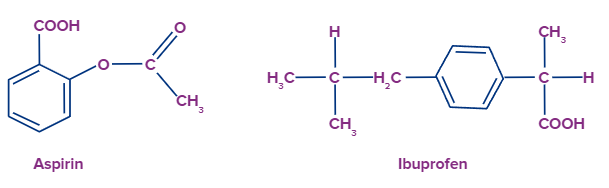
- Aspirin (2-Acetoxybenzoic acid) is a carboxylic acid, and certain people are allergic to it because of its acidity.
- Ibuprofen, a non-aspirin pain medication, is likewise a carboxylic acid.
- Fatty acids are carboxylic acids (triglycerides in the human body) with exceptionally long chains of carbon (C) atoms connected to them. They play a role in the development of fat in the body, as their name implies.
- Malic acid (C4H4O4) (found in apples), tartaric acid (grape juice), oxalic acid (spinach and various sections of the rhubarb plant), and lactic acid (found in milk) are all carboxylic acids that humans consume (sour milk).
- Propionic acid (C3H6O2) and butyric acid (C4H8O2) are two other simple carboxylic acids. The flavour and odour of Swiss cheese are largely due to propionic acid. Butyric acid is not only responsible for the odour of rancid butter, but it also adds to perspiration odour.
- Lactic acid (C3H6O3) is produced in the body's muscles as individual cells digest sugar and perform labour. The weariness felt in the muscles by such short-term use is due to a buildup of lactic acid induced by over-exertion. The lactic acid in the body is gradually converted to water and carbon dioxide as one rests, and the feeling of exhaustion fades.
- Ascorbic acid (vitamin C), C6H8O6, is a carboxylic acid.
- Amino acids, which are carboxylic acids with a nitrogen-containing component termed an amine group in the molecule, are a specific type of carboxylic acid. (contains -NH2 as well as -COOH group.)
- Because proteins are made up of combinations of amino acids, amino acids are extremely significant. Proteins are one of the three major dietary components, along with fats and carbohydrates. Protein makes up a large portion of the human body, including skin, hair, and muscle.
Carboxylic Acids - Significant Applications
Carboxylic acids are compounds that can be made in laboratories or on a large scale (synthesis) from the oxidation reactions of aldehydes, primary alcohols, and hydrocarbons, oxidative cleavage of olefins, base catalysed dehydrogenation of alcohols, or hydrolysis of nitriles, esters, or amides. Organic acids serve an important and diverse role in modern society, as indicated by their numerous applications in medical, agriculture, pharmaceuticals, food, and other fields.
Pharmaceutical medications, polymers, biopolymers, coatings and adhesives are all made from carboxylic acids and their derivatives. They can also be utilised as flavourings, food additives, and antimicrobials.
Food: Organic acids have an essential role in the food industry since they influence organoleptic qualities (such as taste, aroma, and colour) as well as food stability.
- They can be found naturally in fruits and vegetables (citric acid in citrus fruits, malic acid in grapes and apples, oxalic acid salts in parsley and broccoli), or they can be added artificially as acidulants (citric acid), preservatives (lactic acid), emulsifiers (tartaric acid), antioxidants (ascorbic acid), or flavours (propionic acid) in a wide range of human-food products (foods and beverages).
- The monitoring or regular assay of amount and type of organic acids found in foods and beverages give useful information for monitoring fermentation processes, controlling production, storage, and distribution, and detecting probable adulteration.
- Organic acids are well-known as good preservatives, and their antibacterial activity stems from their capacity to switch from an undissociated to a dissociated state depending on the pH of the environment, making them potent antimicrobial agents.
- Certain organic salts (For e.g., calcium and sodium propionate) are used as preservatives in dairy and bakery foods to prevent bacteria and fungi from growing and ruining the product.
- However, some carboxylic acids are advantageous to microorganisms, acting as vitamins for microbial nutrition and assisting their growth (e.g. nicotinic acid, folic acid, or p-aminobenzoic acid).
Medicine and Pharmaceutical Industry: Carboxylic acids play an important part in medicine as well. Organic acids are intermediate metabolites of all major categories of organic cellular components, and their presence in excess in various bodily fluids has been related to the emergence of specific disorders on numerous occasions. The following are the most essential roles carboxylic functionalities play in pharmaceuticals:
- Solubilizer that affects solubility, lipophilicity, and cell penetration (for example, antibiotics and antihistamines).
- Prodrug and/or bioprecursor chemicals that are not biologically active but are transformed into active molecules under certain conditions (e.g. antihypertensive, antithrombotic, or antiviral medicines)
- A pharmacophore is a molecule that has a specific interaction with an enzyme, either activating or inhibiting its biological reaction (e.g. blood cholesterol-reducing drugs, non-steroidal anti-inflammatory drugs).
- Carboxylic acid-containing medicines are widely used in the treatment of pain and disorders in medicine.
Cosmetics: The alpha hydroxy acids (AHAs) are a type of organic acid that makes a significant contribution to the cosmetics industry. This group includes tartaric, lactic, citric, malic and glycolic acids, which are widely used in cosmetics for objectives such as unblocking/cleaning pores, improving skin texture, whitening, anti-wrinkle, and acne therapy.
- Additionally, carboxylic acids such aldobionic acids (ABAs), retinoic acids, vitamin C, and azelaic acid are the most effective antioxidants and anti-aging agents, as well as increasing moisture retention.
- The carboxylic acid-based esters are the most well-known derivatives for their flavours and scents, and they're frequently used in perfumes, deodorants, and air fresheners.
- Saponification (cold process) to make soaps from sodium hydroxide and long chain fatty acids (oils) are very significant in the soap industry.
Geological Processes: Soil development, surface weathering, subsurface porosity production, and ore formation all involve organic acids. Organic acids also have a major and variable effect in soil acidification and mineral weathering, according to a certain research.
Other carboxylic acids, such as phenolic and fatty acids, act as allelochemicals, inhibiting the normal growth of plants and algae, resulting in negative physiological and ecological effects.
Carboxylic Acids - Uses in Various Sectors
- Higher fatty acids are frequently used in soap manufacturing.
- Acetic acid is used as a coagulant in the rubber manufacturing process.
- Carboxylic acids are utilised for a variety of reasons in the rubber, leather, and textile industries.
- Hexanedioic acid is a chemical that is utilised in the manufacture of nylon-6,6.
- Chelating compounds like ethylenediaminetetraacetic acid are widely used in a variety of applications.
- Chemicals with the carboxyl functional group are used to make several polymers.
- For humans, carboxylic acids are the building blocks of essential fatty acids. Omega-6 and omega-3 fatty acids are two of the many examples.
- The majority of carboxylic acids are employed in the production of soft drinks and other foods.
Practice Problems
Q. 1. Which of the following is not an application of formic acid?
a. Textile industry
b. Tanning Leather
c. Coagulation of Rubber
d. Antipyretic medication
Answer: Formic acid or methanoic acid (HCOOH), is used as a coagulating agent for latex coagulation and produces a high-quality natural rubber product. Also, it is used in the textile and leather industry, but not as an antipyretic medicine.
So, option D) is the correct answer.
Q. 2. Which carboxylic acid is used in the manufacturing of Nylon-6,6?
a. Hexanedioic Acid
b. Propanedioic Acid
c. Butanedioic Acid
d. Pentanedioic Acid
Answer: Condensation polymerization of adipic acid with hexamethylenediamine yields the polymer, nylon-6,6. Adipic acid is basically hexanedioic acid.
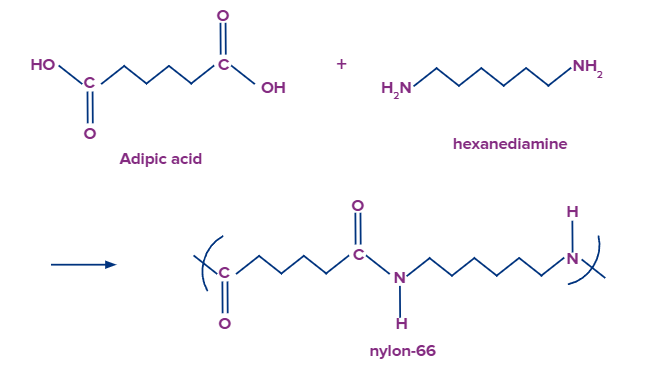
So, option A) is the correct answer.
Q. 3. Commonly used carboxylic acid for marination in culinary purposes is?
a. Acetic Acid
b. Terephthalic Acid
c. Butanoic Acid
d. Adipic Acid
Answer: Acetic acid (CH3COOH) in diluted form (vinegar) is used for marination in cooking.
So, option A) is the correct answer.
Q. 4. Which of the following acids are not used to make soap?
a. Palmitic Acid
b. Stearic Acid
c. Linoleic Acid
d. Tartaric Acid
Answer: The first three acids are naturally obtained oils and fats and are very commonly used in soap making. Tartaric acid is obtained in grapes and is not an ingredient for soap making (saponification).
So, option D) is the correct answer.
Frequently Asked Questions - FAQ
1. How are carboxylic acids significant to cosmetics and skincare industry?
Answer: Carboxylic acids are the most potent antioxidants and anti-aging agents, as well as moisture-retention agents used in making various skincare products. Alpha-hydroxy acids, like glycolic acid and other carboxylic acids like aldobionic acids (ABAs), retinoic acids, vitamin C, and azelaic acid, are commonly used in cosmetics and skincare having anti-aging and moisturising properties.
2. Which carboxylic acids are used as medicines?
Answer: Organic acids are utilised in numerous medications in the pharmaceutical business, such as aspirin, ibuprofen and phenacetin. They work mainly as a non-steroidal, anti-inflammatory class of drugs.
3. What are some ester derivatives of carboxylic acid that are used as a substitutive artificial fragrance?
Answer: Esters have a sweet and sometimes even fruity smell and are hence used as artificial fragrances in the food industry and in perfumery. For example, esters like benzyl acetate smells like pear or peach, ethyl methylphenylglycidate smells like strawberry, and ethyl pentanoate mimics the scent of apple.
4. What is the negative impact of high triglycerides (obtained from carboxylic acids) in our body?
Answer: A triglyceride is defined as an ester constituted from three fatty acids and glycerol. Triglycerides, as well as dietary fat, are the major components of body fat in humans and other animals.
Excessive triglyceride levels lead to artery hardening or thickening (arteriosclerosis), which manifolds the risk of stroke, heart attack, and heart disease. Extremely high triglycerides can potentially induce severe pancreatic inflammation (pancreatitis).


