-
Call Now
1800-102-2727
Electroplating Process - Introduction, Components, Metals Used, Benefits and Thick Layer Formed by Electroplating, Practice Problems, FAQs
There is a girl whose name is Nikita. Nikita was having lunch in her new shiny lunchbox and notices that the bottom of the lunch box is coated with a shiny substance. She wears a gold bracelet every day which looks shiny yellow. Upon taking a metal scraper and rubbing her bracelet, she notices that the yellow colour weans off. She now realizes that the lunch box and her bracelet aren’t actually made of gold; they are just coatings.
Many people in our country can’t afford such expensive jewellery, but everyone has a right to be happy, so now with the help of electroplating, less expensive jewellery is also available in the market.
The process of coating one metal over another metal is preferred when huge costs are incurred in making a substance. For instance, making a gold necklace is expensive compared to making a silver necklace. The latter can be coated with gold to give it an aesthetic appearance and not mention, protect it against corrosion or other forms of degradation.

A process known as electroplating is going to help us achieve this; as the name itself suggests, it makes use of electricity to facilitate the coating of one metal over another.
In this article, we will explore electroplating in detail and learn how the electroplating process work!
TABLE OF CONTENT
- What is Electroplating?
- Components of the Electroplating Process
- Metals used in Electroplating Process
- Electroplating Process
- Benefits of electroplating
- Practice Problems
- Frequently asked questions-FAQs
What is electroplating?
The technique of adding a layer of metal to the surface of another metal is known as electroplating. Typically, this is done to stop metals from rusting, and in the case of gold and silver plating, it is done to make the decoration look more beautiful and affordable. An electric current is introduced into a solution during the electroplating process, causing the solution to separate into its component ions and deposit on the electrode's surface.
Components of the Electroplating Process:
The electroplating process consists of four key steps:
1. Anode: The metal that will create the coating layer on the surface of another electrode is this positively charged electrode.
2. Cathode: The metal surface that will receive plating is this negatively charged electrode.
3. Electrolyte Solution: This fluid encourages the concomitant mobility of ions and the passage of electricity. Salt of the metal from which the anode is produced often serves as the electrolyte solution.
4. Power supply: The process of electroplating involves introducing electricity into the solution to start a chemical reaction. Therefore, a good power source will be crucial to the electroplating system.
Metals used in the Electroplating Process:
The commercial use of electroplated materials is extremely widespread; everything from the cables we use to the jewelry we wear is electroplated. Gold, silver, platinum, chromium, cadmium, brass, copper, nickel, and other metals are used in electroplating. The utilization of the metals depends on what function the electroplated layer will fulfill. The most widely utilized metals include copper, nickel, gold, and a variety of alloys.
Different alloys are utilized for electroplating because they increase the material's strength and hardness and stop the metal in question from corroding. An example is the alloy Zamak 5, which is composed of 95% zinc, 4% aluminium, and 1% copper.
Electroplating Process:
The electroplating process consists of an anode and a cathode. The cathode is the terminal where electrons undergo reduction. The metal to be gold plated is made as the cathode. On the other hand, the anode is a gold plate which is to be deposited on the cathode. Both the electrodes are kept immersed in an electrolytic solution, which is made from a salt of the metal taken. Reduction occurs on the cathode and oxidation occurs on the anode. Factors which affect the process of electroplating are –
1) The voltage applied by the external circuit.
2) The surrounding bath temperature and chemical composition of the electrolyte.
3) The time duration of electroplating.
4) The spacing between the anode and cathode.
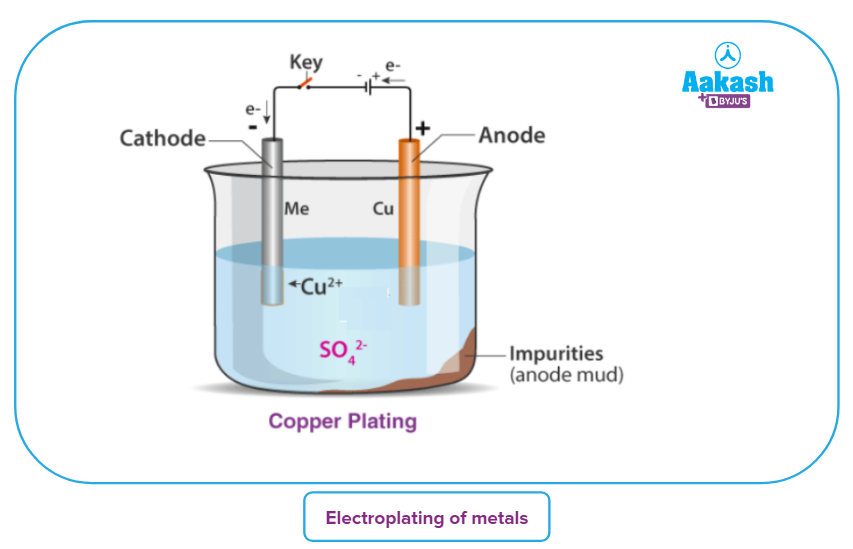
Let us take the example of copper plating. The anode is made of a block of pure copper metal which can be coated on the cathode. The latter is an impure copper plate which needs to be provided with a protective coating. The electrolyte is dilute copper sulphate solution (CuSO4). When current is passed through the electrolyte, it dissociates into Cu2+ and SO42-. The process goes on till the current is passed. The impurities get deposited as anode mud at the bottom of the vessel.
Cu2+ ions are reduced to metallic copper at the cathode. While at the anode, Cu gets oxidized to
Cu2+. This process keeps on going on till the electric current is stopped.
CuSO4 -> Cu2+ + SO42- (electrolyte dissociating)
Cu2+ + 2e- -> Cu (at the cathode)
How thick is the layer formed by electroplating?
The thickness of the electroplated layer can change depending on what function it will fulfill. Thicknesses often fall between 0.5 microns and 20 microns. To achieve comprehensive protection for avoiding corrosion, the layer must be thick.
Benefits of electroplating:
The importance of electroplating in a metal's protection has been emphasized throughout the article. In order to ensure protection, electroplating has a number of advantages.
1. Plating: The electroplated metal layer serves as a barrier between the original metal and harmful environmental factors. This increases a product's ability to be sustainable.
2. Enhanced Electrical Conductivity: If the material has to have better electrical conductivity, plating it with silver or gold can be an affordable alternative.
3. Provides Heat Resistance: Zinc and nickel alloys work well as heat-resistant barriers and stop any material from being damaged too soon.
4. Appearance: Commercially, gold, silver, and platinum-plated jewelry are well-liked due to their reasonable costs and sturdiness.
5. Used for smoothening tools: Coating a surface with nickel can minimize friction and stop wear and tear on the equipment instead of using a lubricant
Practice Problems:
Q1. What is the electroplating process' founding principle?
A. Saturation
B. Neutralization
C. Esterification
D. Hydrolysis
Answer: D
Solution: Through the process of electroplating, a metal is plated on top of another in the presence of metal salt (in aqueous solution). The final result of this process is the water molecule. Hydrolysis is hence the basic idea of electroplating.
Q2. Which one of the following is not an electroplating application?
A. Coating of metal
B. Metal protection
C. Extraction of metal
D. Decorative purposes
Answer: C
Solution: Through the process of electroplating, a metal is plated on top of another in the presence of metal salt (in aqueous solution). As a result, it is utilized for coating other metals and for aesthetic purposes. It does nothing with the extraction of metal..
Q3. The method of altering a metal's characteristics is known as ____________.
A. Electrolysis
B. Electro less plating
C. Electrodeposition
D. Electroplating
Answer: C
Solution: Electroplating coats the other metals with a thin layer of metal without changing their characteristics. However, electrodeposition is a procedure that modifies the metal's properties and makes the coating permanent.
Q4. You may make saucepans by electrolyzing________________
A. Galvanized zinc
B. Copper
C. Chromium
D. Nickel
Answer: B
Solution: A thin layer of copper and a thick coating of copper are both used as anodes in the production of saucepans. The appropriate metal to be coated is positioned in a metal salt solution, and the standard electrolysis procedure is then carried out.
Frequently Asked Questions-FAQs:
Q1. In what acid is electroplating done?
Answer: Methanesulphonic acid is used in the electroplating and metal finishing industries. Over the past ten years, methanesulphonic acid has steadily supplanted fluoroboric acid as the preferred electrolyte for the electrodeposition of tin and tin-lead solder on electronic devices.
Q2. How long does a typical metal electrolyte last?
Answer: The electrolyte can remain after the electroplating procedure for up to three hours before it degrades naturally. Decomposition is the term used to describe this electrolyte degradation process.
Q3. Who has discovered this method of electroplating?
Answer: When Luigi Brugnatelli first learned that metals can change electricity from one form to another within themselves in the late 1980s, he got the concept of electroplating. This concept sparked the development of electroplating.
Q4. What will happen if a system's electroplating conditions are exceeded?
Answer: The requirements of temperature and pressure must be met for electroplating to proceed in a steady state. The procedure stops if the circumstances go beyond or in excess of what is required.



