-
Call Now
1800-102-2727
Ozonolysis Mechanism of Alkenes and Alkynes, Practice Problems and Frequently Asked Questions-FAQs
Ozone, as we all know, is a highly reactive gas made up of three oxygen atoms. It occurs in the upper and lower atmospheres of the Earth. Ozone has different effects on life on Earth depending on where it resides in the atmosphere.
The ozone layer also protects ourselves and other living things from the sun's harmful UV radiation.

But do you know it's also employed in chemical reactions?
Yes, ozone is employed in the oxidation of numerous organic molecules, which is referred to as ozonolysis.
Let’s discuss this ozonolysis in detail!
Table of Contents:
- Introduction
- Ozonolysis mechanism of Alkene
- Ozonolysis mechanism of Alkyne
- Practice Problems
- Frequently Asked Questions
Introduction
It is possible to define ozonolysis as a chemical reaction that uses an ozone molecule to cleave unsaturated bonds. Unsaturated chemical compounds like alkenes and alkynes are among those that are subject to ozonolysis.
Ozonolysis Mechanism of Alkene:
One bond exists in alkenes. When alkenes are oxidized in the presence of O3 molecule, products such as ketones, aldehydes, or carboxylic acids are formed in this reaction.
Example:
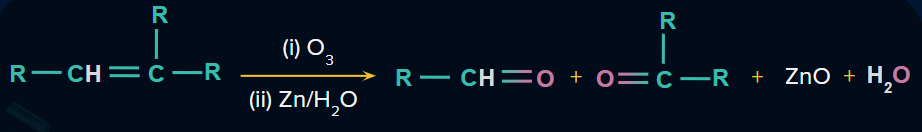
Consider the following example to better understand the reaction mechanism of ozonolysis in an alkene.
Mechanism:
Three steps are involved in the ozonolysis reaction mechanism.
Step 1: Attack of ozone molecule
The ozone molecule has one positively and one negatively charged oxygen atom. The exchange of electrons between alkene having bond and ozone molecules result in a cyclic structure known as molozonide.

Step 2: Ozonide intermediate formation:
Molozonide is quite unstable. In molozonide, one bond still holds the carbon atoms together. The next reaction transforms molozonide into an intermediate through a rearrangement that is relatively stable, ozonide. The carbon atoms in ozonide are joined by oxygen atoms.

Step 3: Carbonyl compound formation:
(i) Reductive Ozonolysis:
Ozonide is further reduced in the presence of dimethyl sulphide or zinc dust. As a result of this reaction, two products are formed. We can see a double bond between carbon and oxygen atoms in both products. This is an example of an ozonolysis reaction. Ozonolysis is the lysis of a double bond in the presence of an ozone molecule. The end products of ozonolysis of alkenes would be ketones, aldehydes, or carboxylic acids.
- Zn and H2O
- (CH3)2S and H2O
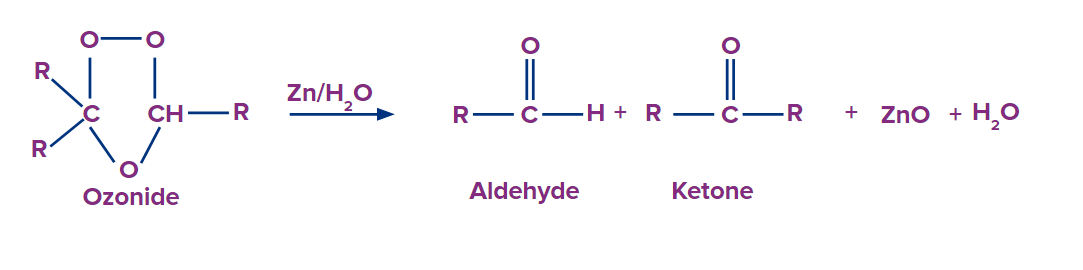
(ii) Oxidative ozonolysis:
This type of alkene ozonolysis involves the addition of an ozone molecule to an alkene to form an ozonide, which is then oxidized to smaller oxidized molecules such as acids or ketones. The following is the reagent used in oxidative ozonolysis:
- H2O
- H2O2
The oxidative ozonolysis reaction can be described as follows:

Note: In oxidative ozonolysis, if formic acid is formed, it will further decompose into carbon dioxide and water.
HCOOHCO2+H2O
Ozonolysis Mechanism of Alkyne:
Alkynes have two bonds. The triple bond of alkynes is oxidized in the presence of an O3 molecule. End products such as diketones and acid anhydrides are formed in this reaction. In the presence of water, the acid anhydride hydrolysis to produce carboxylic acids.
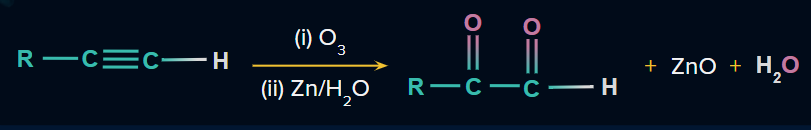
Mechanism of ozonolysis in an alkyne:
Three steps are involved in the ozonolysis reaction mechanism.
Step 1: Ozone molecule attack:
The ozone molecule has one positively and one negatively charged oxygen atom. The exchange of electrons between alkyne having bond and ozone molecule results in a cyclic structure which is unstable.
Step 2: Ozonide intermediate formation:
This intermediate will experience further rearrangement to create a stable ozonide.

Step 3: Carbonyl compound formation:
Later on, ozonide will react as per two conditions as given below
(i) Reductive Ozonolysis:
Ozonide is further reduced in the presence of dimethyl sulfide and zinc dust. As a result of the reduction reaction, one bond between two carbon atoms breaks, resulting in the formation of a diketone molecule. We can see a double bond between carbon and oxygen atoms in both products. The end product of ozonolysis of alkynes would be diketones.
- Zn and H2O
- (CH3)2S and H2O

(ii) Oxidative ozonolysis:
This type of alkyne ozonolysis involves the addition of an ozone molecule to an alkyne to form an ozonide, which is then oxidized to smaller acids. The following is the reagent used in oxidative ozonolysis:
- H2O
- H2O2
The oxidative ozonolysis reaction can be described as follows:
Practice Problems:
Q1. Predict the product when ethyne undergoes ozonolysis in the presence of methyl disulfide.
a. Glyoxal
b. Formic acid
c. Formaldehyde
d. Acetic acid
Answer: (A)
Solution: In this given question, the addition of an ozone molecule to ethyne during reductive ozonolysis results in the formation of an ozonide, which is then rearranged to give dialdehyde, Ethane-1,2-dial which is also known as Glyoxal.
Hence, the correct answer should be option (A).

Q2. Predict the product when propyne undergoes ozonolysis in the presence of hydrogen Peroxide.
a. 2-oxo propanal
b. Carbon dioxide
c. Acetic acid
d. Both (B) and (C)
Answer: (D)
Solution: In this problem, the addition of an ozone molecule to propyne during oxidative ozonolysis produces an ozonide, which is then further oxidised to give acids acetic acid and formic acid. Formic acid being unstable decompose to give carbon dioxide and water in the presence of an oxidizing agent. Hence the product of ozonolysis of propyne is Acetic acid and carbon dioxide.
As a result, the option should be the correct response is (D).
Q3. Oxidative ozonolysis of alkene results in the formation of
a. Carboxylic acids
b. Ketones
c. Carbon dioxide
d. All of these
Answer: (D)
Solution: In this problem, the addition of an ozone molecule to alkene during oxidative ozonolysis produces an ozonide, which is then further oxidised to give acid. If the alkene is terminal, it will give Formic acid, which is being unstable and decompose to give carbon dioxide and water. If the alkene is substituted, it will give ketone as a major product.
As a result, the correct response is (D).

Q4. On reductive ozonolysis of X gives acetone and propanal. Predict X
a. 3-methyl pent-3-ene
b. 2-methyl pent-2-ene
c. 3-methyl pent-2-ene
d. 2-methyl pent-3-ene
Answer: (B)
Solution: There is no such molecule exist like 3-methyl pent-3-ene and 2-methyl pent-3-ene. Hence option (A) and option (D) are inappropriate.
In option (C), 3-methyl pent-2-ene will give butan-2-one and Acetaldehyde and in option (B), 2-methyl pent-2-ene will give Propionaldehyde or propanal and Propan-2-one or acetone.
Hence the correct option is (B).

Frequently Asked Questions-FAQs:
1. What chemical applications does alkene have?
Answer: Alkenes have a wide range of applications in the manufacturing industry. As starting materials, they are used in the synthesis of alcohols, plastics, lacquers, detergents, and fuels. The most important alkenes in the chemical industry are ethene, propene, and 1,3-butadiene.
2. What are the industrial applications of ozonolysis?
Answer: Bleaching makes use of ozonolysis. Ozonolysis is a technique used in wastewater treatment. Ozonolysis is a chemical reaction that produces carboxylic acids, aldehydes, and ketones.
3. What is the role of zinc in ozonolysis?
Answer: Zinc dust is used withinside the ozonolysis method to prevent the carbonyl molecule from in addition oxidation. By inhibiting the molecule from forming extra oxygen bonds, Zn halts the process.
4. Is ozone a nucleophile or an electrophile?
Answer: The properties of molecular ozone include those of a dipole, an electrophilic agent, and a nucleophilic agent. The dipolar nature of the ozone molecule can result in 1-3 dipolar cycloadditions on unsaturated bonds, culminating in the creation of primary ozonide.


