-
Call Now
1800-102-2727
Sawhorse Projections: Conformers, Sawhorse Projection, How to Write Sawhorse Projection, Advantages, Practice Problems & FAQs
Which is your favourite pizza?
Obviously having pizza is not a healthy option but still, we do eat it and share it with our loved ones.
What if I tell you that, till now you all are eating pizza in the wrong way?

Yes, let me tell you why?
Pizza is basically made up of 3 things
- Crust
- Base
- Toppings

The main taste of the pizza is its toppings but whenever we eat pizza, we take the slice from the box and eat it in a way that the crust portion first touches our tongue rather than the toppings. Your tongue contains taste buds.
So how to enjoy the toppings more?
Just flip the pizza and eat it in a way that the toppings first touch your tongue.
Isn’t it interesting that just by changing the orientation we can have different results?
We all know that most organic molecules are oriented in 3 dimensions. All atoms and groups have occupied a position in the spatial arrangement of atoms. Now if the orientation is changed then definitely the activity of the molecule will also get changed.
Hence, to understand this spatial arrangement, 2-dimensional drawing of these 3-dimensional molecules is done mostly in three ways. They are Fisher projection, Newman projection and Sawhorse projection.
In this concept page, we will learn more about sawhorse projection.
Table of Contents
- Conformers
- Conformations of Ethane
- Sawhorse Projection
- How to Write Sawhorse Projection
- Advantages of Sawhorse Projection
- Practice Problems
- Frequently Asked Question-FAQ
Conformers
All organic compounds, except the first member of every homologous family, have carbon-carbon sigma (𝞂)bonds. Along the internuclear axis of the C-C bond, the sigma molecular orbital is symmetrical around it and is not perturbed by rotation on its axis. Free rotation around the C-C single bond is thus possible. As a result of this rotation, atoms are arranged differently in space and can interact with one another. Conformations, conformers, or rotamers are the terms used to describe these spatial groupings of atoms that can be transformed into one another by rotating around a C-C single bond.
This means that by rotating around C-C single bonds, alkanes can adopt an endless variety of conformations. It's important to keep in mind that rotation around a C-C single bond is not entirely free. Due to the mild repulsive nature of neighbouring bond electrons, there is a minor energy barrier of about 1 to 20 kJ mol-1. that prevents the free rotation around the sigma bond. Torsional strain is a term used to describe this kind of repellent interaction.
Conformations of butane:
The 2 and 3rd carbon atoms of butane (C4H10) have, a carbon-carbon single bond. Each of these carbon atoms is attached to two hydrogen atoms and one methyl group. Keep one carbon atom immobile and move the other one across the C-C axis in accordance with the "ball and stick" representation of butane. The hydrogen atoms and the methyl groups linked to each carbon atom can be arranged in an endless number of different spatial configurations as a result of this rotation. Conformational isomers are these (conformers). As a result, butane can exist in an endless number of conformations.
Eclipsed; When all the hydrogen atoms and methyl group or attached groups to the carbon atoms are on the same plane, the conformer is called an eclipsed conformer.
Staggered or anti: When all the hydrogen atoms and methyl groups or attached groups to the carbon atoms are on opposite planes, the conformer is called a staggered conformer.
Skew or gauche - Skew or gauche conformers are intermediate conformations that exist between two standard conformations.
It is important to note that the bond lengths and angles are constant throughout all conformations. Sawhorse and Newman projections can be used to depict eclipsed and staggered conformations.
Sawhorse Projection
Sawhorse is a wooden structure resembling the trunk of a horse supported by four legs used in pairs to support wooden logs for sawing(cutting).
In organic molecules, the -C-C- bond is comparable to the central strong wooden log of the sawhorse. The three bonds attached to each carbon of the molecule is similar to the legs of the saw horse.
The difference is that-
- In the sawhorse model of organic molecule we have three more sigma bonds(instead of two legs), each at a 120° angle to each other for both carbon atoms.
- All three bonds can be rotated along the connecting -C-C- bond axis
- The structure is seen from an angle to the central axis, such that all the constituents are visible.
How to Write Sawhorse Projection
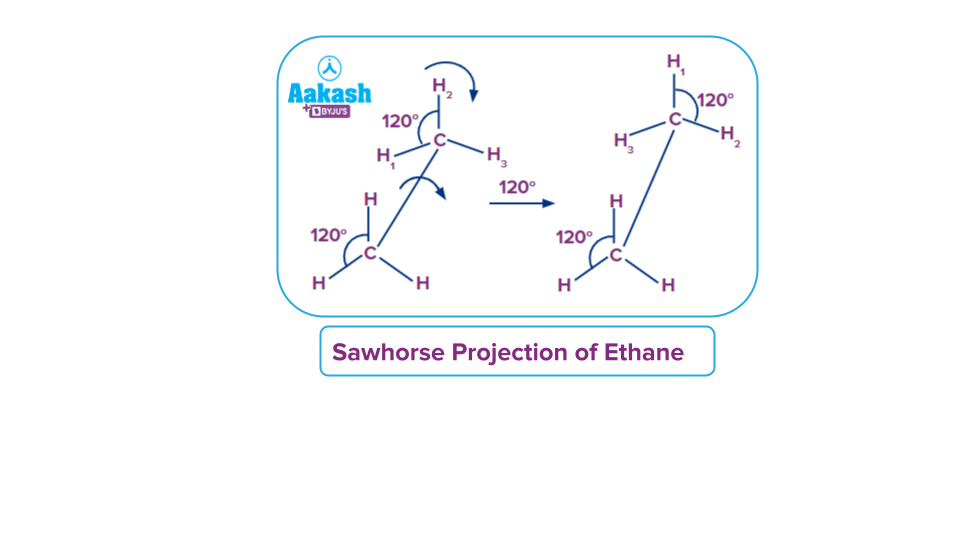
Let’s consider the ethane molecule. C2H6
There are two carbon atoms and 6 hydrogen atoms. Each carbon is attached to 3 hydrogen atoms.
- To represent it in sawhorse projection, draw a line sideways(at some inclination).
- Place the two carbons of interest at each end of this line, so that the connecting line represents the sigma bond.
- Now draw three lines at 1200 an angle to each other and place atoms/groups at the end of the the lines. For example, in the case of ethane place 3 hydrogen atoms on lines drawn on each carbon atom.
Advantages of sawhorse projection
- Easy to visualize all the atoms or groups concerned
- Easy to compare two such structures and check whether they are superimposable or not
- Easy to differentiate structures as enantiomers or diastereomers.
Recommended Video: Projection formulae and their inter-conversion | CHEMISTRY | JEE | Concept of the Day | SM Sir
Practice Problems
Q1. What is the molecular formula of the structure presented in sawhorse projection?
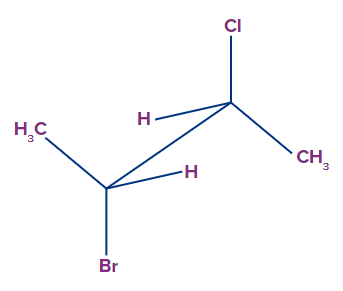
a. C4H8BrCl
b. C2H6BrCl
c. C3H8BrCl
d. C4H6BrCl
Answer: (A)
Solution: C4H8BrCl
In the given structure, We have caron atom each at the ended of the central straight line.
In the left side carbon, we have, 1C + CH3 + Br + H or C2H4Br
In the right side carbon, we have, 1C + CH3 + Cl + H or C2H4Cl
Addibng both we get the molecular formula of the compound as C4H8BrCl.
Hence, option A is the correct choice.
Q2. Which of the following strains could exist in the ethane molecule's eclipsed form?
a. Only Van der Waal strain
b. Van der Waal strain
c. Torsional strain
d. Both A and B
Answer: (C)
Solution: Because there is no torsional strain or Van der Waal strain, the staggered conformation is the most stable type of ethane conformation. While Van der Waal strain, which is noticed due to the bulkiness of the molecule but won't be seen in an ethane molecule because the size of a hydrogen atom is small, is present in the most unstable form of the ethane conformation, known as an eclipsed form, in which there is a torsional strain that is present due to bond pair-bond pair repulsion as dihedral angle corresponds to 0 degree.
Q3. Which of the following forms can be produced by rotating the molecule along the butane molecule's C2-C3 bonds?
a. Staggered
b. Gauche
c. Eclipsed
d. All of the above
Answer: (D)
Solution: When the butane molecule is rotated at an angle greater than 60 degrees, all three forms will be visible. The gauche form, in which two methyl groups are at an angle of 60 degrees adjacent to one another, is the next most unstable form after the eclipsed form because both the methyl groups are situated behind one another in two separate carbons. The most stable version is the one where the methyl groups are spaced 180 degrees apart from one another.
Q4. What is the angle between the hydrogen atoms attached to the carbon atoms in a C2H6 molecule represented in a sawhorse projection?
a. 600
b. 1200
c. 900
d. 450
Answer: (B)
Solution: A view of a molecule looking sideways of a specific carbon-carbon bond is known as a sawhorse projection. The desired C-C molecular axis is viewed sideways in a sawhorse projection, with each carbon atom at each end of the axis connected to its corresponding atom or group at a 120° angle.
Hence, Option B is the correct choice.
Frequently Asked Questions-FAQs
Q1. How do we assess the conformers' relative stability?
Answer: The conformers exhibit torsional strain and van der Waal strain. In general, van der Waal strain, which is present because of the group's steric crowding, causes the conformation to be unstable and has higher energy than the conformer that exhibits torsional strain, which is present because the group is attached to the front and back carbons and is repelled by their bonds, creating bond-pair bond pair repulsion. The type of strain a conformer contains is used to determine its relative stability. It is determined experimentally by the energy difference needed to go through the barrier.
Q2. What exactly is a system with rotational restrictions?
Answer: A rotation-restricted system is one in which the bond cannot be rotated along the atom to which it is bound. The pure orbital participates in the bonding in the case of double and triple bonds, and it cannot be rotated until the bond is broken.
Q3. What do you understand by ‘dihedral angle’?
Answer: The angle between two groups linked to two separate carbon atoms in the Newman configuration is known as the dihedral angle. Rotating the valencies along the carbon results in a change in the dihedral angle, which affects the conformer's stability.
Q4. How can the stability of the gauche and staggered conformation be assessed
Answer: Since the dihedral angle in the staggered form is equal to 180 degrees, torsional strain and Van der Waal strain are absent, making the staggered conformation generally more stable than the gauche form. However, in situations when intramolecular H-bonding is seen, the gauche form's stability outperforms the staggered form.


