-
Call Now
1800-102-2727
Reverse Osmosis: Introduction, Principle, Working Process, Advantages, Disadvantages, Applications, Practice Problems & FAQs
Seamen used to say “water water everywhere but not a drop to drink '' Similar such situations have come to exist in urban cities, where it is impossible to get natural potable water. Water from all sources, whether municipal, ground or bore, contain different amounts of dissolved solids in them. The concentration of such salts is mostly over the limits, set for a quality portable water.
Every urban household has started using reverse osmosis (RO) plants to get portable water. If so, what is reverse osmosis and how is it useful to get portable water?
Reverse osmosis has become a necessary technology for human survival and you will learn more about it.
Table of content:
- Reverse osmosis
- Reverse osmosis principle
- Reverse osmosis process working
- Advantages of reverse osmosis
- Disadvantages of reverse osmosis
- Applications of reverse osmosis
- Practice problems
- Frequently asked questions
Reverse osmosis:
In Osmosis you might have learned that salt solutions naturally draw pure water to their side and application of pressure to the salt solution side will force pure water out of a salt solution through a semipermeable membrane. This exactly is the reverse osmosis.
In reverse osmosis water purification method, a semipermeable membrane is used to keep all the ions, dissolved salts and undissolved compounds, to be held and removed from the pressured side of the membrane letting only the pure solvent to the collecting chamber on the other side of the semipermeable membrane.
Reverse osmosis is one of the oldest and most widely used separation techniques, mostly for water purification. The procedure was primarily used for seawater desalination in 1950, when the entire process was somewhat slow and limited to specific laboratories. However, following much research and technological improvements, substantial advances were made, particularly in the field of polymers and the creation of effective membranes.
Today, many people all over the world utilise this approach to purify water for industrial, residential, commercial, and scientific uses.
Reverse Osmosis principle:
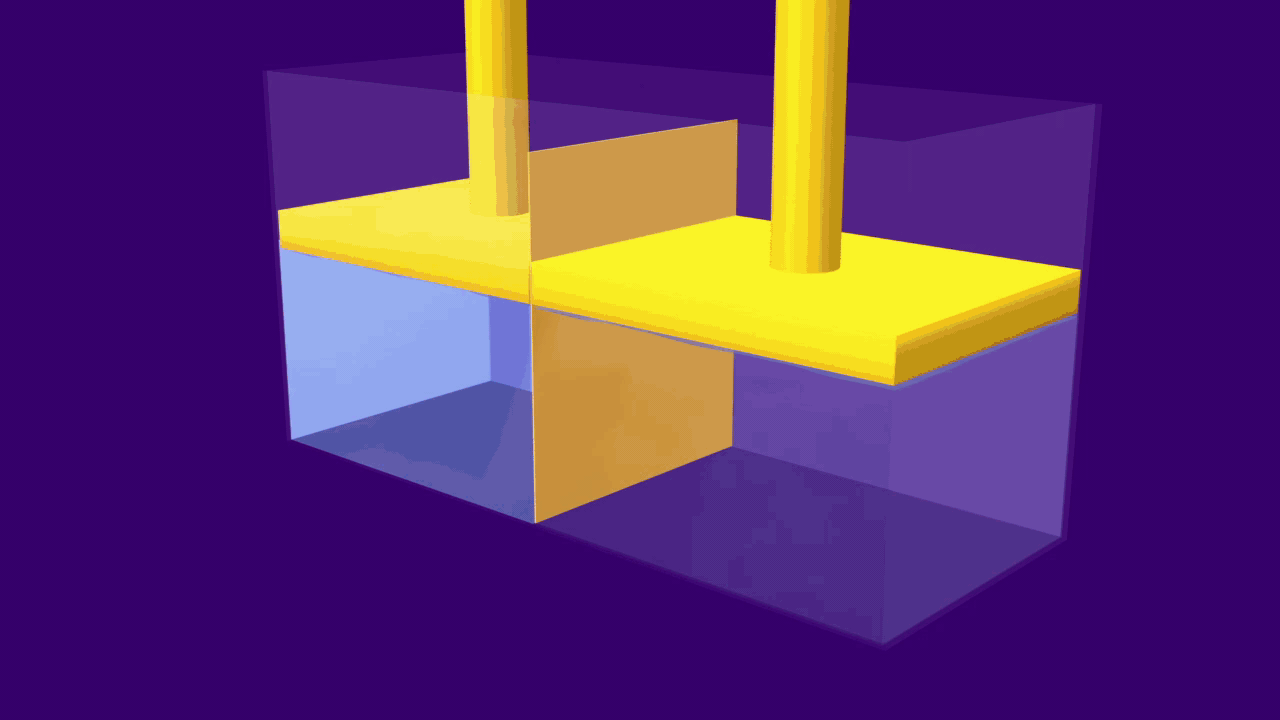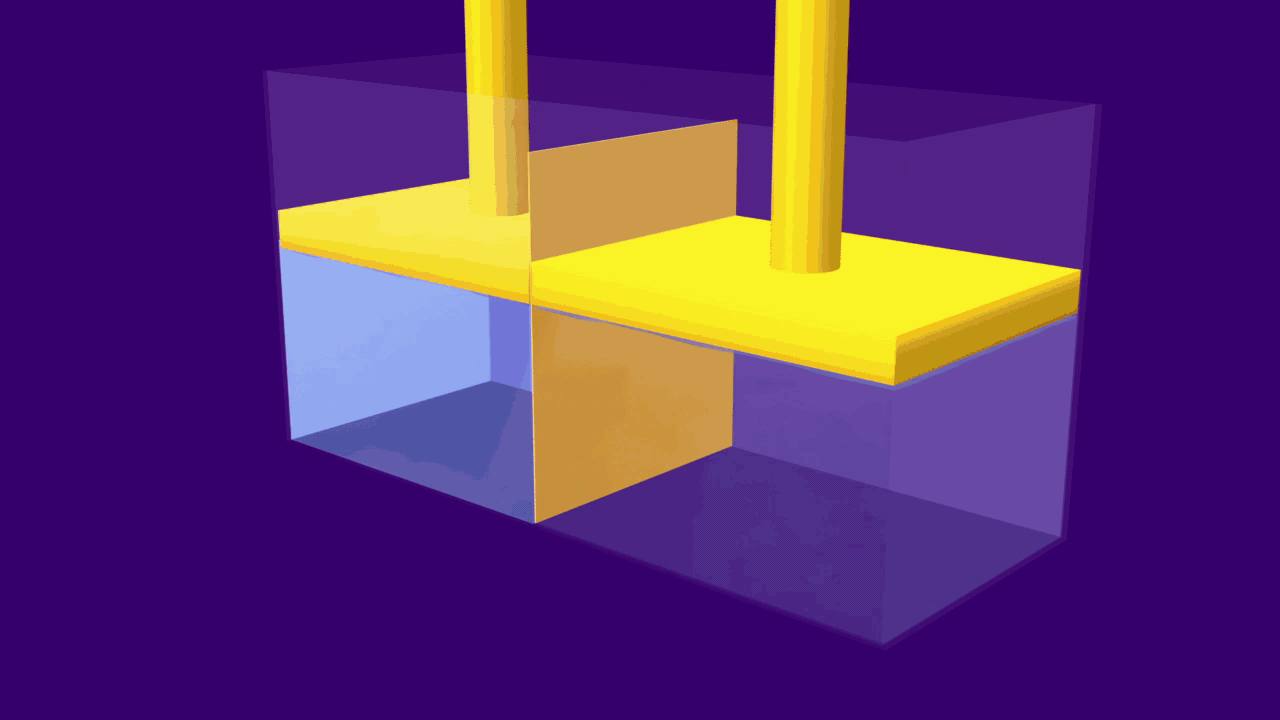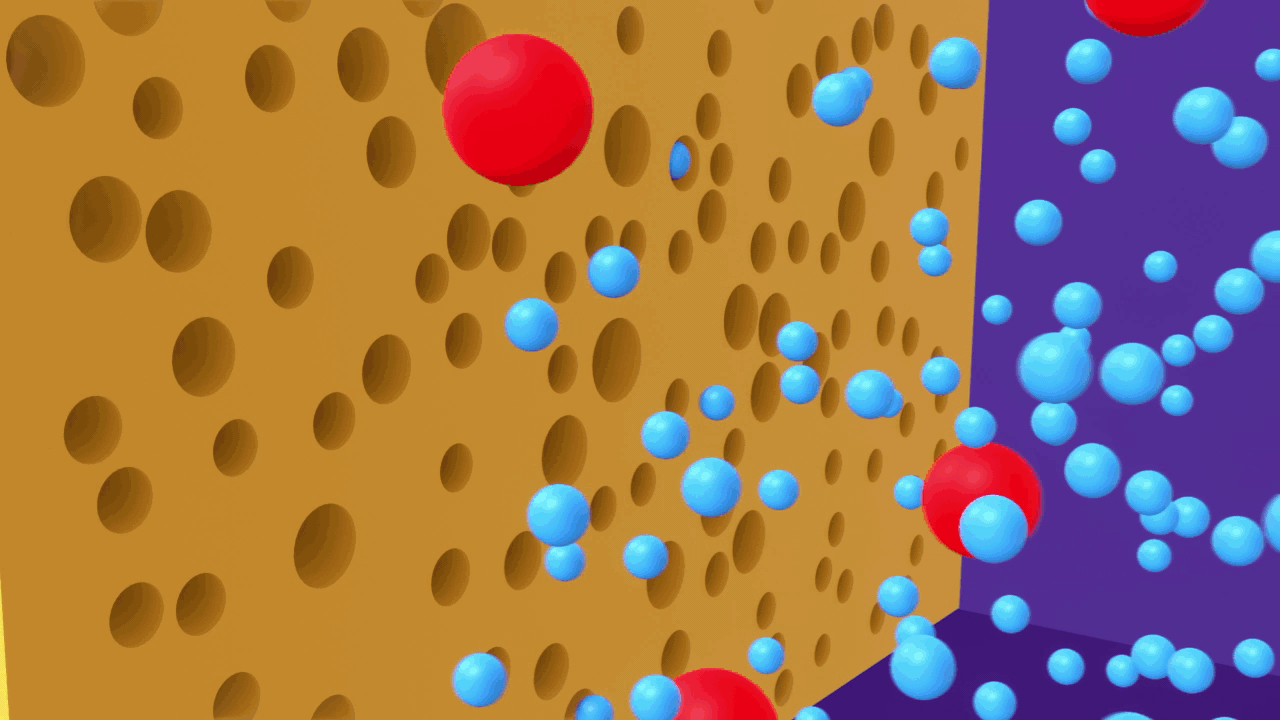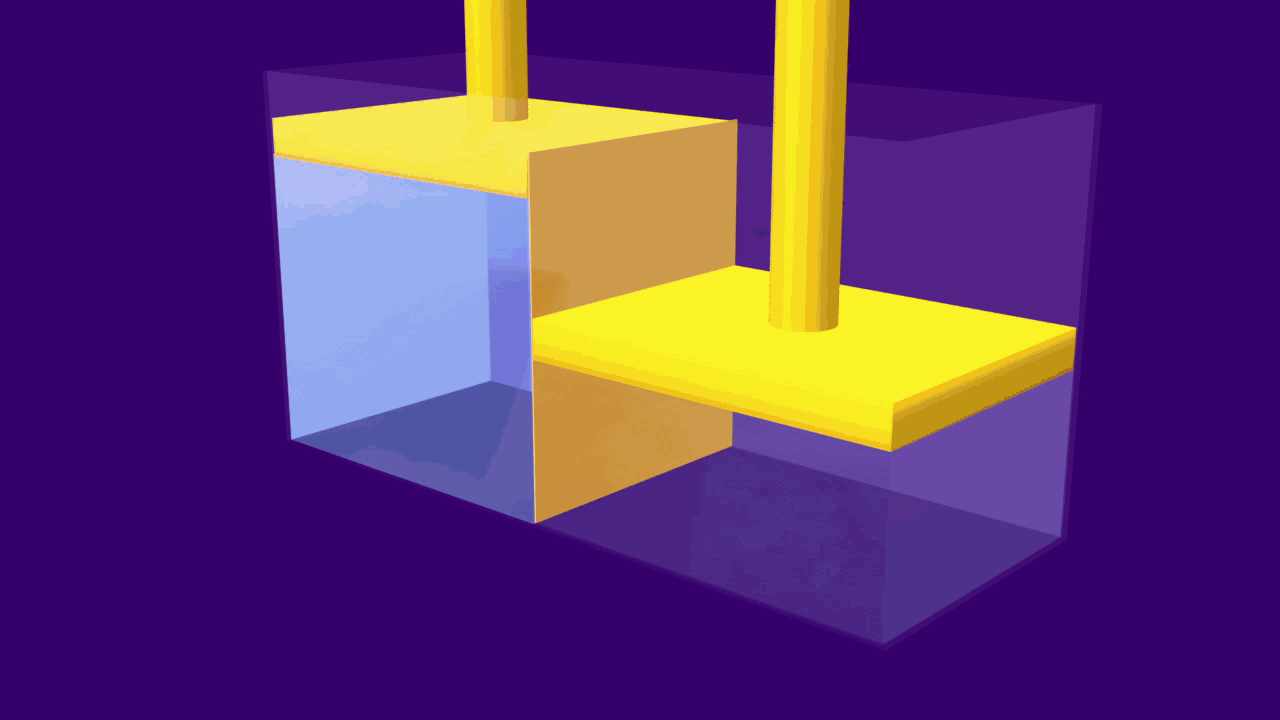
When an external pressure greater than the osmotic pressure is applied, the flow of solvent molecules can be pushed to move in the reverse direction of osmosis, from solution to pure solvent. This is known as reverse osmosis.
![]()
In this technique, pressure is applied to the solution side of, a semipermeable membrane. Large molecules in the solution are stopped by the semipermeable barrier, the pure solvent, on the other hand, can pass through the membrane and can be collected for use.
As a result, the molecules of the solute begin to concentrate on one side of the membrane. The solution levels alter to some extent during the reverse osmosis process.
Reverse osmosis process working:
A simple experiment can be used to explain the process of reverse osmosis. Freshwater and a concentrated aqueous solution are taken. The semipermeable membrane is placed between the two solutions, separating them. The pressure is applied to the membrane's end, i.e. to the concentrated solution. As a result, water molecules will begin to move through the membrane. Contaminants, on the other hand, will remain on the aqueous solution side, while water molecules will flow to the freshwater side.
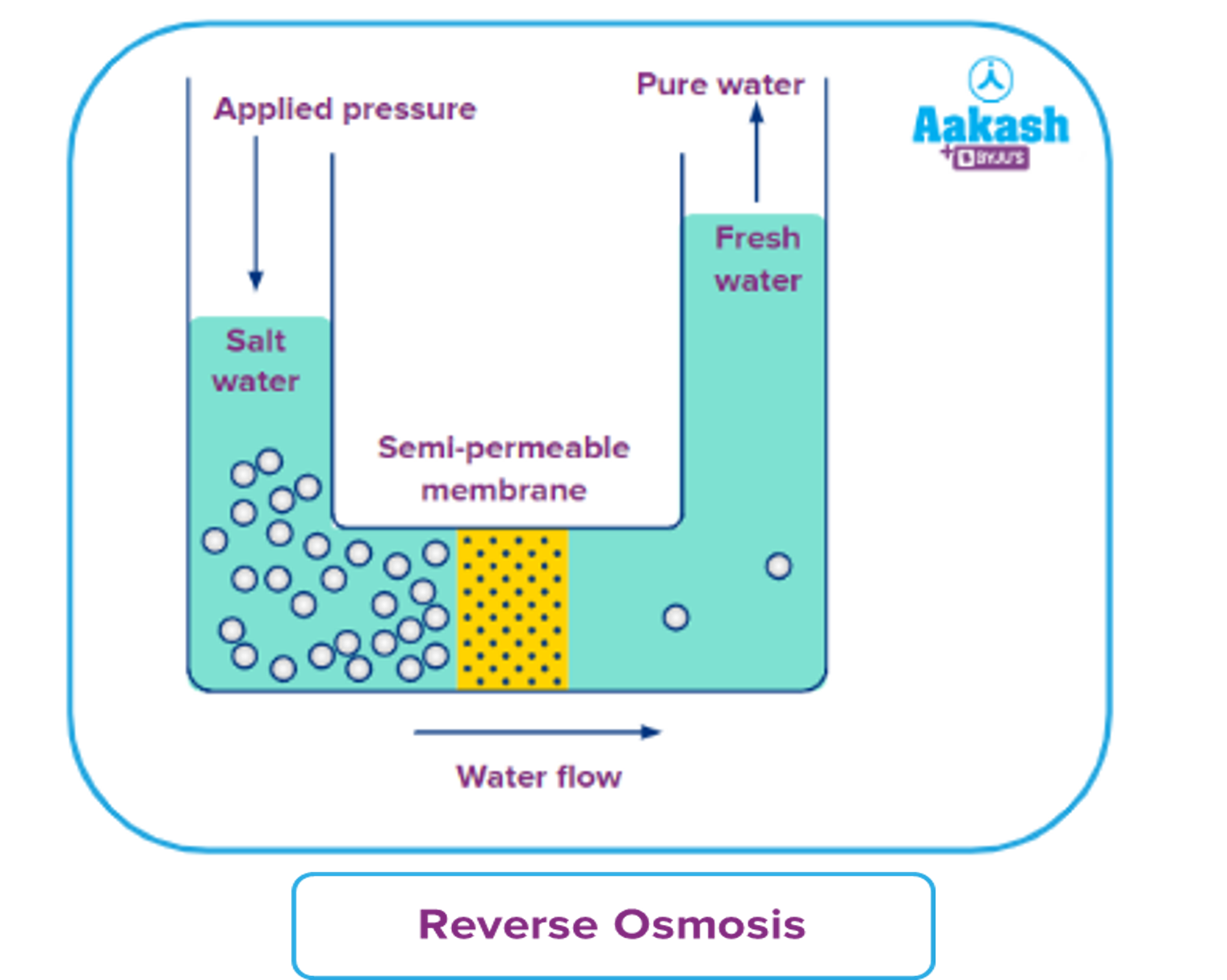
The reverse osmosis process is depicted in the diagram. When the concentrated solution is subjected to increased pressure, the water molecules begin to move across a semipermeable membrane. The contaminants, however, are unable to pass through the barrier.
Advantages of reverse osmosis:
The following are some of the benefits of the reverse osmosis technique.
- It is the most effective way of softening water.
- All ion particles will be blocked by the semipermeable barrier.
- The system's maintenance is really simple.
- It provides us with clean and pure water by filtering out any impurities.
- The intact membrane rejects bacteria, viruses, and pyrogen materials. In this regard, the quality of RO water is comparable to that of distilled water.
- The available RO systems are small and take up minimal space.
- The entire system, including the membrane, has a useful life of more than two years.
- To cleanse water, this method does not utilise any chemicals.
- The RO system has a very low energy requirement.
- RO systems are completely automated and may start and stop on their own.
Disadvantages of reverse osmosis:
The following are some of the drawbacks of the reverse osmosis technique.
- Sometimes reverse osmosis causes the entire system to clog.
- It requires regular filter replacements and maintenance.
- A reverse osmosis system is expensive to install.
- When it comes to residential applications, the entire process is rather slow because of the low pressure used.
- The technique has no effect on water disinfection. To disinfect the water, you will need an additional technique along with reverse osmosis..
- The system can be harmed by hard water.Any little microorganism can pass through the damaged membrane.
- The applied pressure must be greater than the osmotic pressure for the system to function.
- The RO system is not self-sustaining.
- Cost effectiveness depends on the development of strong and cheaper semipermeable membrane
Applications of reverse osmosis:
Reverse osmosis is a popular method of water filtering in both home and commercial settings. Aside from that, it has numerous applications in a variety of industries. Some of these are listed below.
- Because municipal water is utilised in both households and commercial purposes, reverse osmosis is employed to filter dissolved and contaminated impurities along with infectious microorganisms and help save portable water..
- Ocean offers an unlimited and perennial source of salt water for humanity. The ocean or sea is the only source of water in many locations. Hence more and more reverse osmosis plants are being commissioned to desalinate the sea water and get pure water from sea water both for domestic and commercial utility.
- Reverse osmosis is commonly used in the automobile sector for car washes and equipment cleaning to remove hard spots and protect the vehicle's surface.
- It has crucial applications in medicine.
- It is used in the food industry for the concentration of juices, milk, and other liquids.
- It is used to provide safe drinking water for the community water supply.
Practice problems:
Q.1. Among the following, which is not used as a semipermeable membrane?
(A) Poly Methyl sulphate
(B) Cellulose acetate
(C) Polyamide polymer
(D) Poly Methyl acrylate
Answer: (A)
Solution: Poly Methyl sulphate is not a semi-permeable membrane material. Semi-permeable membranes are made of cellulose acetate, polyamide polymer, and polymethyl acrylate.
Q.2. The reverse osmosis process is also known as
(A) Hyper-filtration
(B) Double-filtration
(C) Double-osmosis
(D) Hyper-osmosis
Answer: (A)
Solution: The reverse osmosis method is often known as super or hyper-filtration. When subjected to a hydrostatic pressure larger than the osmotic pressure, a solvent travels through a porous membrane in the reverse direction of natural osmosis.
Q.3. The basis of reverse osmosis is
a) The osmotic pressure is greater than the hydrostatic pressure
b) The osmotic pressure is equal to the hydrostatic pressure
c) The hydrostatic pressure is greater than the osmotic pressure
d) Osmotic pressure does not exist
Answer: (C)
Solution: The principle of reverse osmosis is that hydrostatic pressure is greater than osmotic pressure. When subjected to a hydrostatic pressure larger than the osmotic pressure, a solvent travels through a porous membrane in the reverse direction of natural osmosis.
Q.4. Semi-permeable membranes are selective membranes that do not allow dissolved _______ particles to pass through.
(A) Solvent
(B) Solute
(C) Anhydrous
(D) Saturated
Answer: (B)
Solution: Semi-permeable membranes are selective membranes that do not allow dissolved solute particles to flow through. It is a biological or synthetic polymeric membrane that allows specific chemicals or ions to diffuse through it.
Frequently asked questions:
Q1. What contaminants are eliminated by reverse osmosis?
Answer: The method of purifying water by removing impurities is known as reverse osmosis. This technique aids in the removal of impurities such as bacteria, viruses, salts, and other undesirable elements, making the water safe to drink for all. These pollutants are removed by passing these substances across a RO membrane. The size and charge of the substance determine whether or not it is removed from water. However, some solvents, such as carbon dioxide, cannot be removed by reverse osmosis.
Q2. Is Reverse Osmosis Water Safe? Give an explanation.
Answer: Depending on the system, reverse osmosis water might be either good or bad. Reverse osmosis water removes all important components such as iron, magnesium, calcium, and fluoride. Fluoride is significantly more vital for children in order to avoid cavities. If your reverse osmosis system removes all of these necessary components from water, the water becomes unsafe to drink. It is best to select the system accordingly, as minerals do not always indicate that they are contaminants. They are also good for the body.
Q3. What differentiates osmosis from reverse osmosis?
Answer: Osmosis is a type of diffusion in which water molecules move through a semipermeable membrane from a high to a low water potential area. Reverse osmosis, on the other hand, is the passage of water molecules through a semipermeable membrane against a concentration gradient.
The process of osmosis does not require the use of energy, however reverse osmosis does.
Osmosis is a natural process, whereas reverse osmosis is used commercially for a variety of purposes.
Q4. What does the reverse osmosis process do not remove?
Answer: Typically, reverse osmosis is used to remove impurities from water that are harmful to human health, such as lead, dissolved salts, mercury, iron, and calcium. As a result, it removes all turbidity caused by bacteria and dissolved chemicals. However, the procedure fails to remove potentially dangerous pesticides as well as other volatile components and compounds. Chlorine and radon are two of these substances. Herbicides and agricultural treatment agents such as fungicides are also examples of contaminants that cannot be removed by reverse osmosis.


