-
Call Now
1800-102-2727
Reduction of Nitriles – Nitriles, Isonitriles, Their Properties, Preparation of Amines and Aldehydes by the Reduction of Nitriles
Have you ever questioned the purpose of using gloves in chemical labs?
We use gloves in chemistry labs because it is hazardous for us to expose our skin directly to some chemicals.

Can we handle the chemicals in the lab with woollen gloves on?
Of course not! These gloves are only intended to keep our hands warm during winter. The gloves we use in a chemistry lab are different from the ones we typically wear throughout the winter. Nitrile rubbers are typically used to make medical and laboratory gloves. In addition to this crucial use, nitriles can be reduced to create amines, which have a wide range of additional uses. Therefore, in order to employ nitriles more effectively, it is crucial to learn more about them.
We will learn about nitriles, isonitriles, their characteristics, and how to make amines and aldehydes by reducing nitriles on this concept page.
TABLE OF CONTENTS
- Nitriles – Definition
- Nitriles – Properties
- Isonitriles – Definition
- Reduction
- Preparation of Amines by the Reduction of Nitriles
- Preparation of Aldehydes by the Reduction of Aldehydes
- Practice Problems
- Frequently Asked Questions – FAQ
Nitriles – Definition
In organic chemistry, nitriles are the organic substances also known as cyano compounds. This group of organic compounds have the functional group cyanide in their formula, which is -C N: . Cyanides are inorganic substances containing a -CN group.
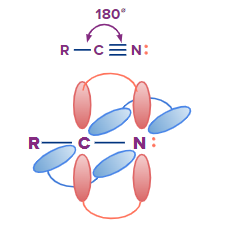
Nitriles – Properties
- They are colourless solids or liquids with distinctive odours.
- Their boiling temperatures range from 82 to 118 °C.
- They have strong dipole-dipole interactions as well as weak van der Waals forces of dispersion between their molecules.
- They exhibit strong electronegativity and polarity.
- They are extremely soluble in water, and their solubility decreases as the chain length increases.
Isonitriles – Definition
An organic compound with the functional group -N+C- is known as an isocyanide, also known as isonitrile or carbylamine. Its prefix is isocyano because it is an isomer of the closely related nitrile (-CN).
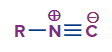
Reduction
According to traditional or earlier conceptions, reduction is a process that involves the addition of hydrogen or any other electropositive element or the removal of oxygen or any other electronegative element.
According to the electronic notion, reduction is the process by which an atom or an ion gains one or more electrons.
Preparation of Amines by the Reduction of Nitriles
In organic chemistry, nitriles, commonly known as cyano compounds, are organic molecules. In which the carbon atom is connected to a functional group known as the cyano group, which is denoted by the functional group (-C N).
By reducing with lithium aluminium hydride, nitriles can be converted to several primary amines. Nitriles, obtained from aldehydes undergo partial reduction to form imine, and this reaction is commonly known as Stephen reduction. Imine on further reduction converts into primary amine in the presence of reducing agents that is Sn/HCl. This reaction is used for the preparation of amines containing one carbon atom more than the starting compound.
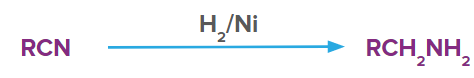
Reduction using H2/Ni
Nitriles produce primary amines in presence of H2/Ni.
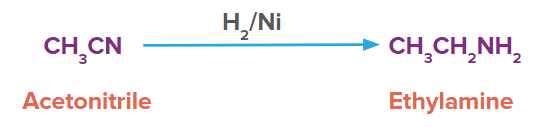
For example, ethyl nitrile in presence of H2/Ni produces ethylamine.
Benzene nitrile in the presence of H2/Ni produces benzylamine.
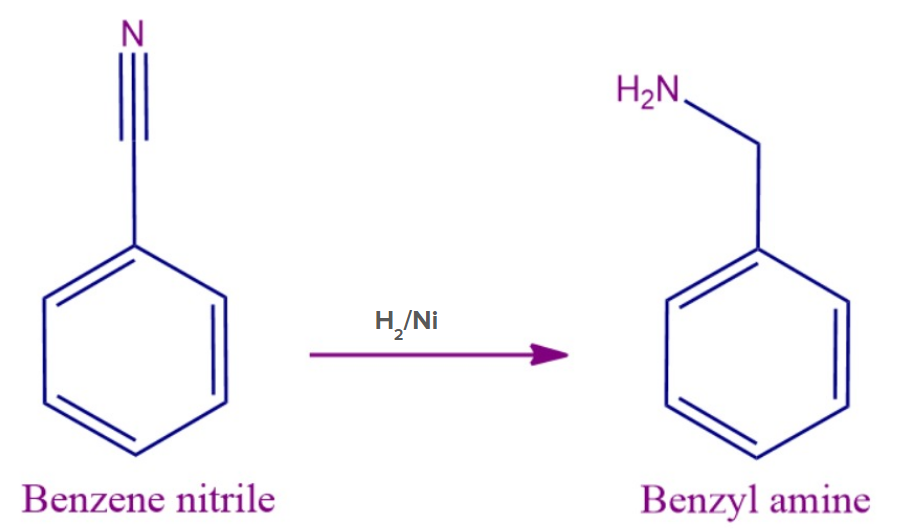
Reduction using LiAlH4
Nitriles produces primary amimes in presence of LiAlH4-THF.
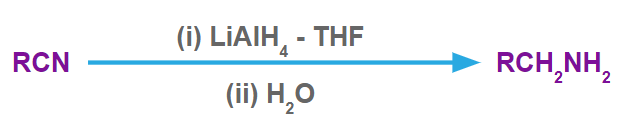
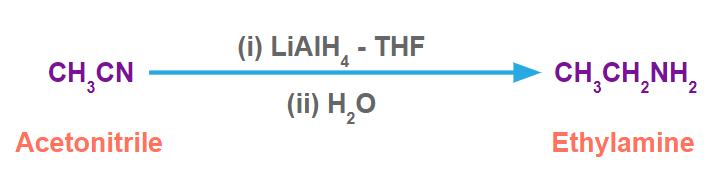
For example, acetonitrile in the presence of LiAlH4-THF produces ethylamine.
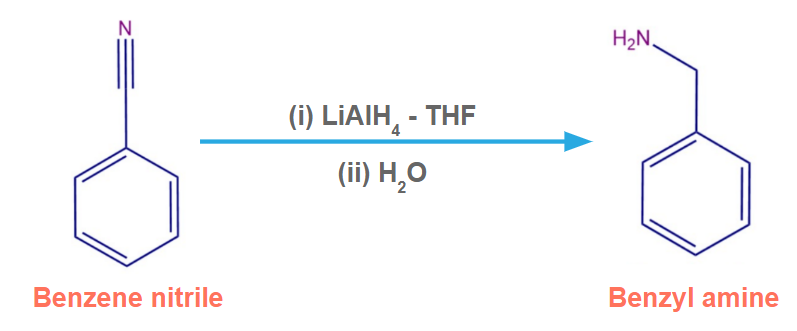
Benzene nitrile in the presence of LiAlH4-THF produces benzylamine.
Reduction using Na(Hg)/EtOH
Nitriles produce primary amines in the presence of Na(Hg)/EtOH.

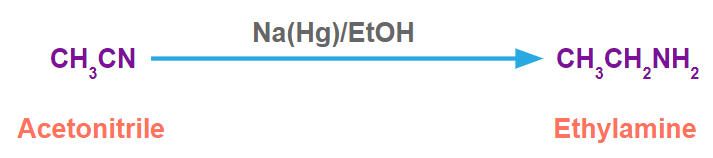
For example, acetonitrile in the presence of Na(Hg)/EtOH produces ethylamine.
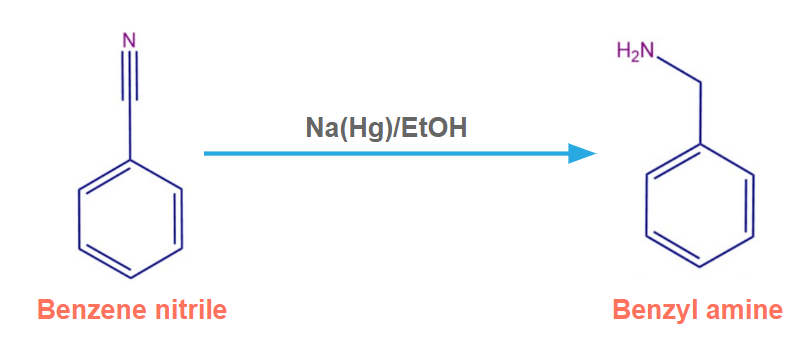
Benzene nitrile in the presence of Na(Hg)/EtOH produces benzylamine.
Preparation of Aldehydes by the Reduction of Nitriles
Reduction using DIBAL-H
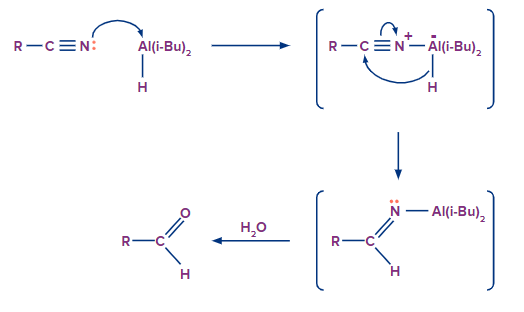
Nitriles are typically converted to aldehydes using the reducing agent DIBAL-H, which stands for diisobutylaluminum hydride. According to the suggested mechanism, DIBAL joins the nitrile through the creation of an N-Al bond to form a Lewis acid-base adduct. The hydride is subsequently transferred to the nitrile's carbon. Aldehyde and ammonia are produced during aqueous workup as desired.
By Stephen’s Reduction
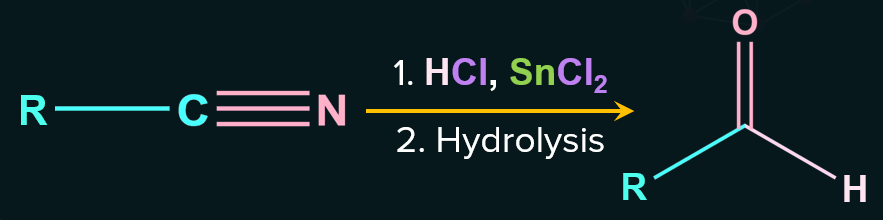
Reduction of cyanide is carried out with acidified stannous chloride (SnCl2/HCl) at room temperature. As a result, imine hydrochloride is formed, which on subsequent hydrolysis with boiling water gives aldehyde.
Practice Problems
1. The carbon atom present in nitrile group is:
- sp3 hybridised
- sp2 hybridised
- sphybridised
- d2sp3 hybridised
Answer: C
Solution: Nitrile group contains a nitrogen atom and a carbon atom that is sp hybridised. The carbon atoms present in the alkyl chain (R) can be sp3 or sp2 hybridised.
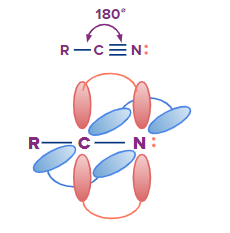
So, option C is the correct answer.
2. Which of the following compounds is formed when benzene nitrile reacts with LiAlH4?
- Benzylamine
- Phenol
- Toluene
- None of the above
Answer: A
Solution: Benzene nitrile in the presence of LiAlH4-THF produces benzylamine.
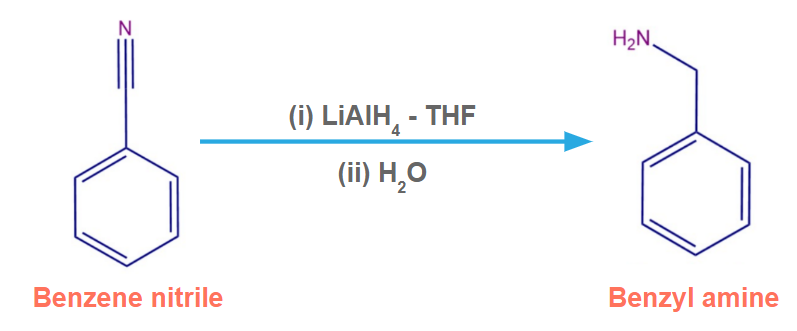
So, option A is the correct answer.
3. The treatment of ethyl nitrile with Sn/HCl produces:
- Ethanol
- Acetone
- Acetaldehyde
- Ethylamine
Answer: D
Solution: Nitriles which are obtained from aldehydes undergo partial reduction to form imine, this reaction is commonly known as Stephen reduction. Imine on further reduction converts into primary amine in the presence of reducing agents that is Sn/HCl. This reaction is used for the preparation of amines containing one carbon atom more than the starting compound. Ethylnitrile gives ethyl amine in presence of Sn/HCl.
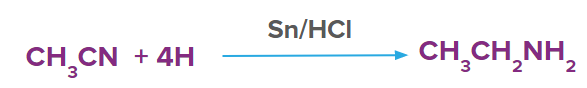
So, option D is the correct answer.
4. Which of the following is not a reducing agent?
- Hydrogen peroxide
- LiAlH4
- Sn/HCl
- Fe/HCl
Answer: A
Solution: One of the reactants of an oxidation-reduction process is a reducing agent, which reduces the other reactant by donating electrons to it. The reduction process cannot take place if the reducing agent does not transfer electrons to another component during a reaction. LiAlH4, Sn/HCl and Fe/HCl are some of the examples of reducing agents. Hydrogen peroxide (H2O2) is an oxidising agent. This reagent oxidises the reactant by itself undergoing reduction.
So, option A is the correct answer.
Frequently Asked Questions – FAQ
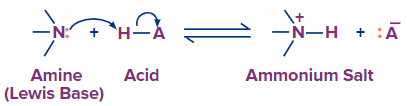
1. What is the nature of amine, acidic or basic?
Answer: Amines are basic and readily react with the electron-deficient hydrogen of acids. Because of the lone pair of electrons on the nitrogen, amines are one of the few neutral functional groups that are regarded as bases. A N-H bond is created when lone pair electrons attack an acidic hydrogen during an acid/base reaction. In the resulting ammonium salt, this gives the nitrogen four single bonds and a positive charge.
Although primary and secondary amines are typically thought of as bases, they are also very weakly acidic. Amines are therefore amphoteric compounds. An amide is the name for an amine's conjugate base.
2. Are nitriles soluble in water?
Answer: Water completely dissolves ethanenitrile, and as the chain length increases, the solubility decreases. Nitriles cannot form hydrogen bonds with other nitriles, but they can with water molecules, which accounts for their solubility.
3. What is nitrile's polarity?
Answer: Polarity in molecules arises due the comparable electronegative difference between two atoms which combine to form a molecule. Nitriles are polar molecules because they contain an electronegative nitrogen. The elctrocnegativity of nitrogen is very high as compared to the adjacent carbon atom. As a result, nitriles frequently have higher boiling points than molecules of comparable size.
4. Why do we observe a colour change in laboratory gloves after a certain use?
Answer: Blue nitrile gloves can occasionally begin to turn yellow after use and also leave yellow stains on the skin. Acids from the skin and sweat reacting with the nitrile are what causes this. The same colour change can also be caused by other substances, such as nitric acid.


