-
Call Now
1800-102-2727
Nitrene - Introduction, Structure and Reactivity, Generation, Important Reactions, Practice Problems, FAQs
Who all else does not love making friends?
Ram and Shyam were two friends who studied together. They played near their home ground and there were other kids also who were their friends. One day, a cousin of ram named Mohan came and get introduced by Shyam.
Now, all three Ram, Shyam and Mohan are familiar with each other. Have you noticed one thing? Without Ram, Mohan and Shyam will become friends. The answer is No. If there is no connectivity or linkage between them, it is a little difficult for a person to get connected with the other one. Ram here behaves as an intermediate between Mohan and Shyam.

A similar thing also happens in chemistry. Organic reactions which are performed need some kind of intermediate. Their intermediates are short-lived and are generated during the reaction.
There are a few types of reactive intermediates. Carbocation, carbanion, free radicals, carbene, nitrene etc are many types of reactive intermediates.
On this page, we will study in detail the intermediate “Nitrene”!
TABLE OF CONTENT
- Nitrene-Introduction
- Nitrene-Structure and reactivity
- Generation of Nitrenes
- Important reactions of Nitrene
- Practice Problems:
- Frequently Asked Questions – FAQ
Nitrene-Introduction:
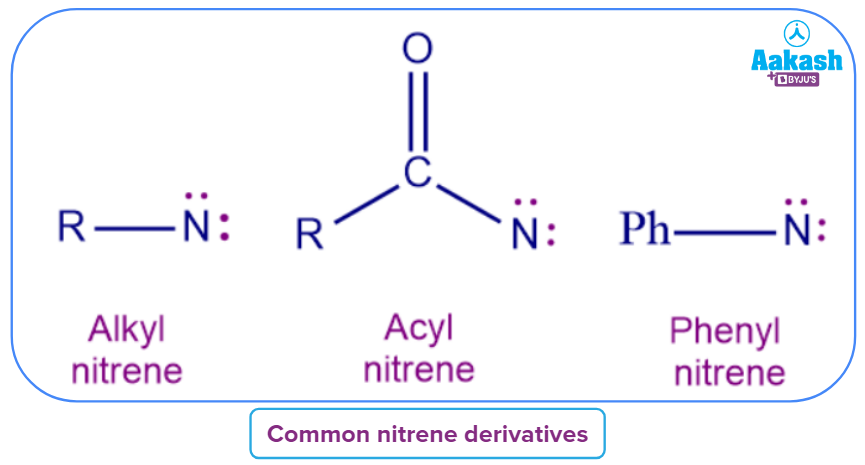
- Nitrenes, or the diatomic molecule N-H and its derivatives, N-R are reactive intermediates that are becoming more critical in both organic and inorganic chemistry.
- When hydrazoic acid or organic azides are heated or exposed to light, nitrene molecules are often produced.
- Since nitrogen possesses six electrons in its valence shell and is thus regarded as an electrophile, nitrenes are the nitrogen counterparts of carbenes.
- The four dots in the depicted structure represent the four nonbonded electrons, of which two are the "typical" lone pair associated with nitrogen.
Nitrene-Structure and reactivity:
Nitrenes are peculiar molecular compounds with strong reactivities. They can be produced and detected similarly to carbenes and are isoelectronic, although they typically have distinct reactivities. The chemistry of nitrenes and carbenes is quite similar in almost every way. According to whether the two nonbonding electrons (the typical nitrogen lone pair remains coupled) have their spins paired or parallel, nitrenes may exist in one of two spin states, similar to carbenes.
On the basis of structure, nitrene is classified under two forms; the p-orbitals of the sp-hybridized nitrogen atom are involved in two virtually degenerate nonbonding molecular orbitals (NBMOs) that make up nitrenes, which have two valence electrons. The p-orbitals are classified as either in-plane or out-of-plane orbitals depending on how they are oriented with the molecule.
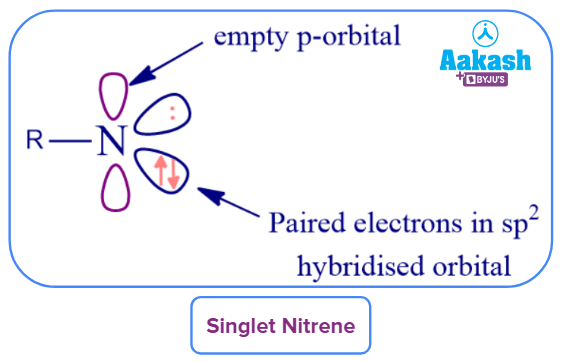
Singlet Nitrene: Singlet nitrenes contain a vacant p orbital and two electrons in a nonbonding sp2 orbital. Following the spin conversion rule will initially result in the production of singlet nitrene during the thermolysis of azides.
Triplet Nitrene: Two parallel-spin electrons will occupy both NBMOs (one sp2 orbital and the other is vacant p-orbital) in the triplet state, reducing the coulombic repulsion between the nonbonding electrons.
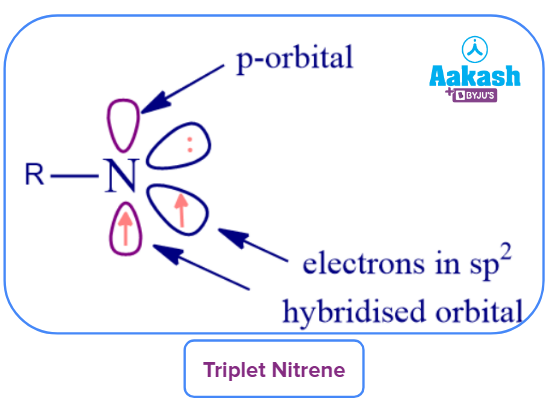
The common ground state for most simple nitrenes is the triplet state, and in this condition, they react slowly. As a result, it is tough to conduct kinetic studies using triplet nitrenes. The triplet nitrenes, however, have the potential to be used as high-spin organic magnetic materials.
Generation of Nitrenes:
Reaction intermediates are crucial components of many organic reaction pathways, such as radicals, diradicals, carbenes, and nitrenes, which need to be understood. The general procedures for producing nitrenes are outlined and are very similar to those employed to produce carbenes.
Due to their high reactivity, nitrenes are not separated but produced in situ, or as reactive intermediates developed during a reaction. Nitrenes are typically produced in one of two ways:
- Through thermolysis or photolysis with the release of nitrogen gas from azides. (This process is comparable to the creation of carbenes from diazo compounds).
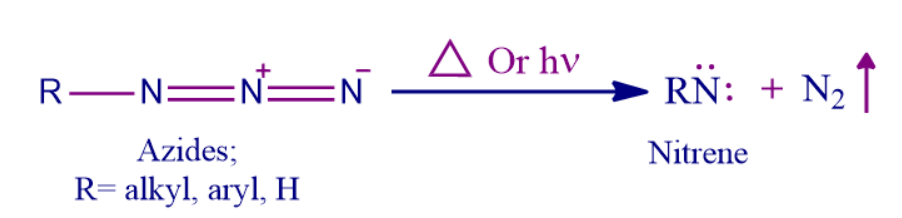
- With the emission of carbon monoxide from isocyanates.
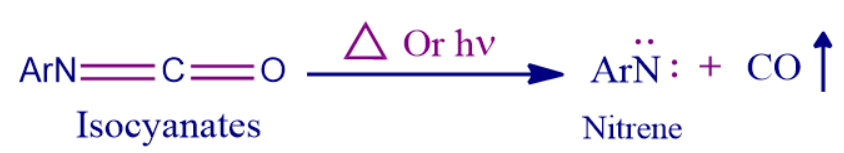
Important reactions of Nitrene:
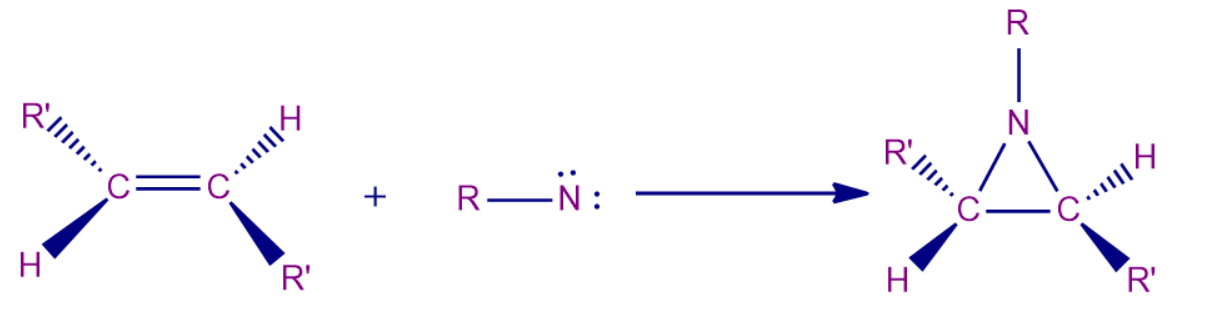
Chemically speaking, nitrenes behave similarly to carbenes in that they both add to and insert into carbon-carbon (C=C) double bonds. They also produce ring closures, extract hydrogen atoms to create primary amino groups, and isomerize to imines. While the triplet nitrenes combine to produce both cis- and trans-aziridines, the singlet nitrenes add carbon-carbon double bonds stereospecifically.
Cycloaddition Reactions of Nitrenes:
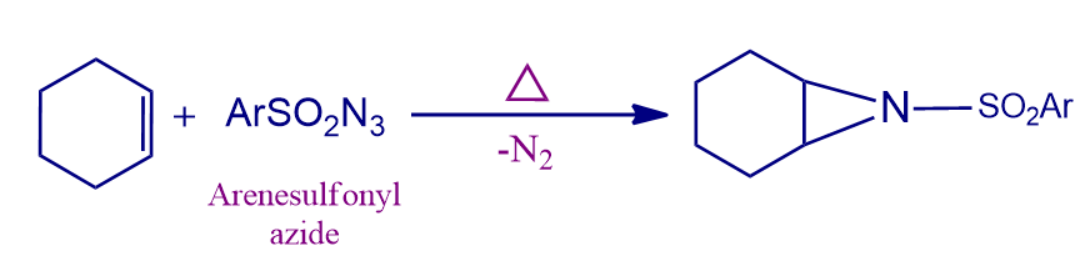
- The 1,2-addition of nitrenes to alkenes results in the synthesis of aziridines. The reaction is most effective with nucleophilic alkenes since nitrenes are typically electrophilic, and the stereochemistry of the resultant aziridine typically depends on the spin state of the nitrene.
- The singlet nitrene is produced by the thermal breakdown of ethyl azidoformates in plain cis-but-2-ene, and it mostly stereospecifically reacts with the alkene to produce the cis-aziridine. As a result, singlet nitrene reacts with maintaining or retaining the original configuration.
- The reaction can be carried out with R = aryl, cyano, EtO2C, and RSO2 as well as other groups.
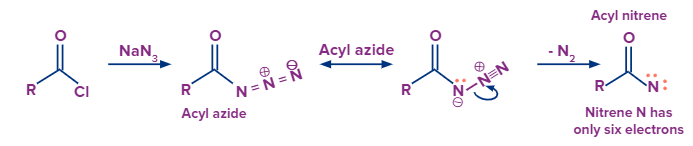
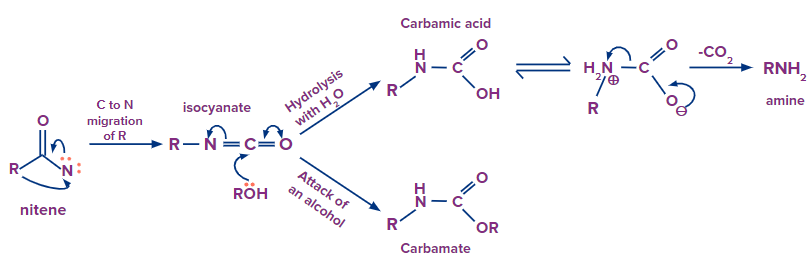
Curtius rearrangement:
- In the Curtius rearrangement, an acyl azide is pyrolyzed to release molecular nitrogen while also undergoing a rearrangement to become an isocyanate (the azides can be created via sodium azide's nucleophilic substitution of an acyl chloride or by the reaction of acyl hydrazides with nitrous acid).
- Azides should be handled carefully since they can disintegrate explosively. Although the isocyanate can be separated by conducting the reaction in an aprotic solvent like chloroform, the isocyanate typically combines with an alcoholic solvent to produce urethane.
- Overall, the Curtius rearrangement transforms an acid chloride with the loss of a carbon atom into an amine.
Here is the process of the formation of intermediate nitrene and the final product:
Practice Problems:
Q1. Nitrenes are isoelectronic with _________and are monovalent nitrogen species.
a. Carbocation
b. Carbanion
c. Radicals
d. Carbene
Answer: D
Solution: Nitrenes are isoelectronic with carbenes and are monovalent nitrogen species. Like a carbene, nitrene nitrogen has only one bond, two lone pairs, and six electrons.
Q2. Nitrenes take part in which of the following rearrangement reactions?
a. Curtius rearrangement
b. Pinacol pinacolone rearrangement
c. The Favorskii rearrangement
d. The benzilic acid rearrangement
Answer: A
Solution: The Curtius rearrangement involves nitrene as a chemical intermediate and changes an acid chloride (or an acid) into an amine with the loss of a carbon atom. Pinacol pinacolone rearrangement involves the rearrangement of carbocation as the intermediate formation. Favorskii rearrangement involves the formation of an enolate whereas benzilic acid rearrangement forms carboxylic acid at its intermediate.
Q3. Two paired spin states for nitrenes in the same NBMO resembles ____________ type of nitrene.
a. Singlet
b. Triplet
c. Both A and B
d. None of the above
Answer: A
Solution: Two paired spin states for nitrenes in the same NBMO resemble Singlet type of nitrene. Singlet nitrenes contain a vacant p orbital and two electrons in a nonbonding sp2 orbital.
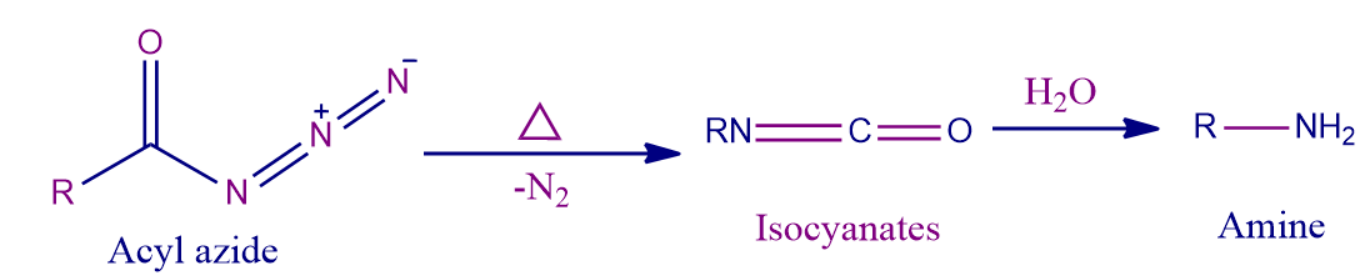
Q4. ____________ is the final product formed in the Curtius rearrangement when hydrolysed with water.
a. Aldehyde
b. Amine
c. Alkanes
d. Acids
Answer: B
Solution: The Curtius rearrangement transforms an acid chloride with the loss of a carbon atom into an amine. In the Curtius rearrangement, an acyl azide is pyrolyzed to release molecular nitrogen while also undergoing a rearrangement to become an isocyanate. Isocyanate when hydrolysed with water give amine as the final product.
Frequently Asked Questions – FAQs:
1. What does an organic chemistry reaction intermediate mean?
Solution: Any responding species in a chemical reaction or mechanism that is no longer a starting material or reactant, has not yet turned into a product and is not in a transition state aids in the reaction's completion.
2. Does nitrene behave as an electrophile or a nucleophile?
Solution: Since nitrenes are often electron-deficient intermediates, and contain only 6 electrons, they react with many kinds of nucleophiles due to their high electrophilicity. In a reaction opposite to their production, tertiary amines, phosphines, sulfides, and sulfoxides all react with nitrenes to create ylides.
3. What are the other reaction intermediates apart from nitrene?
Solution: Carbocations, carbanions, free radicals, carbenes, nitrenes, and benzyne are six different categories of reaction intermediates. These intermediaries are frequently produced when a chemical substance decomposes chemically. A short-lived, highly reactive molecule with high energy is referred to as a reactive intermediate. It will soon change into a more stable molecule when produced in a chemical process.
4. Are insertion reactions possible in nitrene?
Solution: In terms of chemical behaviour, nitrenes act like carbenes in that they combine with carbon-carbon double bonds and insert into C-H single bonds. They also perform ring closures, extract a hydrogen atom to create fundamental amino groups, and isomerize to imines.


