-
Call Now
1800-102-2727
Molar Conductivity and Equivalent Conductivity: Definitions, Relationship Between Molar Conductivity and Equivalent Conductivity, Practice Problems and FAQs:
Are we not calculative and quantitative in our actions? That to, the health conscious people in what and how much they consume per day in terms of calories, proteins vitamins….Don’t you think by tracking the number of calories you take, it will be easier to stay healthy?
Yes, definitely you will remain fit and energetic. There are charts giving these details and packets containing consumables exhibit them on the cover.
In electrolysis also the quantity of electrons or charges (electricity) flowing across electrodes is decided by the nature of the electrolyte. .
If we can find out how much amount of current is carried by a unit volume of electrolyte, it will definitely help us to plan the process in terms of either current needed to get a particular product quantity or vice versa..
So let’s understand some important terms that helps us to calculate electrical flow through the electrolyte and it’s relationship with different variables.
Table of content
- Electrical resistance and conductance
- Conductance (G)
- Conductivity/ Specific conductance
- Molar conductivity
- Equivalent conductivity
- Relationship between molar conductivity and equivalent conductivity
- Practice problems
- Frequently asked questions (FAQs)
Electrical resistance and conductance
Every substance offers resistance to the flow of electricity to a small or large extent. The law that gives the exact value of the resistance is known as Ohm's law. It states that
If to the ends of a conductor is applied a voltage 'E' and a current 'I' flows through it, then the
resistance 'R' of the conductor is E/I. Current is generally measured in amperes, whereas voltage is measured in volts. If one ampere current flows through a conductor when a voltage of one volt is applied to it, the resistance of the conductor is taken as I ohm .
Thus, according to Ohm's law,
V=IR
Where I = current flowing through the conductor
V = potential difference across the conductor
R = resistance offered by the conductor
Conductance (G)
It represents the ease by which current can flow through the conductor.
It is a measure of degree through which conductor can conduct electricity.
Greater the value of conductance, greater the conduction.
It is generally reverse of resistance.
Mathematically, we can write,
Unit of conductance is simen (S)
1 simen (S) = 1 ohm-1 or 1-1 or 1 mho
Resistivity
electrical resistance of a conductor with a unit length and cross-sectional area. Resistivity is a distinctive attribute of any material that may be used to compare different materials based on how well they conduct electric currents. Poor conductors are identified by high resistance.
Where R= resistance of the conductor
A=area of cross section
l= length of the conductor
Conductivity/ Specific conductance
Mathematically
Where
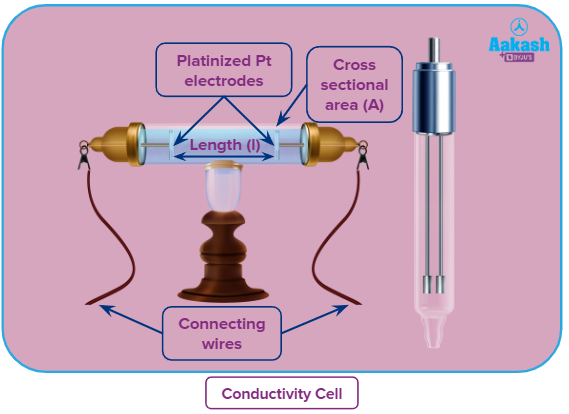
G = conductance of 1 (unit)3 of conductors or 1 (unit)3 of solution.
l = distance between electrodes
A = area of cross section of electrodes
S.I unit of
1 Sm-1 = 1 ohm-1 m-1 = 1 mho cm-1
Common unit : 1 Scm-1 = 1 ohm-1 cm-1 = 1 mho cm-1
Molar conductivity
It is the conductivity of 1 mole of solution of electrolyte dissolved in V volume of solution.
1 (unit)3 = k
V (unit)3 = k.V = Am
As we know
Molarity =
Since n = 1
Hence,
If the unit of is in Sm-1
If the unit of is in S cm-1
S.I unit of molar conductivity
Common unit of molar conductivity
Equivalent conductivity
Conductance of 1g eq. of conductors/electrolyte dissolved in V volume of solution,
1g eq. = amount of substance deposited/liberated by 1 mole of electrons/ 1F
As we know,
Normality
Hence,
Valence factor (Z) for ionic solids
Number of electrons released or accepted in 1 mole of compound is the valance factor of that respective compound.
For example:
S.I Unit of equivalent conductivity
Common unit
Relationship between molar conductivity and equivalent conductivity
Practice problems
Q1. If specific conductivity of N/40 KCl solution at 250 C is 0.0013825 ohm-1 cm-1 and resistance of a cell containing this solution is 300 ohms. calculate the cell constant.
- 0.41475 cm-1
- 4.1475 cm-1
- 41.475 cm-1
- 0.041475 cm-1
Answer: (A)
Solution: We know that
Q2. 1 normal solution of a salt placed between two platinum electrodes 4.0 cm. Apart and of area of cross-section 8.0 sq. cm. has a resistance of 12.5 ohms. calculate the equivalent conductivity of solution.
- 4.0 S cm2 eq-1
- 400 S cm2 eq-1
- 20 S cm2 eq-1
- 40 S cm2 eq-1
Answer: (D)
Solution:
First we need to calculate the specific conductivity.
Given date :
l = 4.0 cm, a = 8.0 cm2 and R = 12.5 ohms
Conductance
Cell constant
Now we can calculate equivalent conductivity
Here c = 1 N, k = 0.04 ohm-1 cm-1
Q3. The electrical resistance of a column of 0.025 M NaOH solution of diameter 10 cm and length
100 cm is 1.11 x 103 ohm. Calculate the molar conductivity of the solution.
- 45.90 S cm2 mol-1
- 4590 S cm2 mol-1
- 4.590 S cm2 mol-1
- 0.4590 S cm2 mol-1
Answer: (A)
Solution: (a) calculation of resistivity
Electrical resistance of the solution, R=1.11 x 103 ohm
Area of cross section of the column
Length of the column (l)=100 cm
Applying the formula
Q4. What will be the valence factor (Z) of KCl solution?
- 2
- 3
- 1
- 5
Answer: (C)
Solution:
Valence factor is defined as the number of electrons released or accepted in 1 mole of compound is the valance factor of that respective compound.
KCl --> K+ + Cl- here Z = 1
Frequently asked questions (FAQs)
Q1. Is the term molar conductivity refers to a constant term?
Answer: Molar conductivity is the conductance property of a solution containing one mole of electrolyte or a function of a solution's ionic strength or salt concentration. Since concentration of the solution can change, conductivity of the solution also will change and will not be a constant.
Q2. Whenever there’s a dilution in the solution, conductivity decreases. Why?
Answer: The conductance of ions present in a unit volume of a solution is called conductivity. The number of ions per unit volume decreases with dilution. As a result, the conductivity drops.
Q3. Why does solutions conduct electricity?
Answer: Because current is carried forward by the ions, the conductivity of a solution is directly proportional to the number of ions present in a unit volume of the solution.
Q4. What impact does temperature have on the molar conductivity?
Answer: The molar conductivity of an electrolyte rises along with the interaction of the ions as the temperature rises.


