-
Call Now
1800-102-2727
Modern Periodic Law – Moseley’s Experiment, The Modern Periodic Law, Characteristics of the Modern Periodic Table

Observe the image given above. The products in the market are arranged in an organised manner so as to reduce the fuss when one of them is required. What would happen if they are not arranged in an organised manner?
If they are not arranged in an orderly manner, we could not find them easily when they are required. So, it is better to arrange things in an orderly manner so as to utilise them to the fullest extent.
This is true in almost all cases, including chemistry. We come across a lot of elements in chemistry, 118 to be precise. The most recent elements are artificially synthesised and do not exist in nature. Efforts are made by scientists to further discover new elements. With so many elements, it is difficult to study the chemistry of each element and the compounds they form separately. So, it is essential to classify and organise these elements so as to understand them to the maximum extent. In the earlier days, scientists looked for a systematic manner to organise the information of all the elements such that the elements possessing similar properties can be simultaneously studied. The first significant effort was made by Mendeleev.
Mendeleev’s periodic table was a huge breakthrough! Its predictive powers were amazing, but it had its flaws. Hydrogen was placed among the alkali metals though it also showed the properties of halogens. Elements like Iodine and Tellurium were completely out of order. The atomic weight of Iodine is less than tellurium but still, it was placed before. Also as time went on, more of these elements were turning up. So, Mendeleev’s work still needed some polishing.
After roaming around from one era to another and having an idea of how different scientists….from Lavoisier to Mendeleev and Moseley….contribute to the idea of sorting elements, it's time to introduce the final and the latest version in which the elements are arranged….in a tabular form….so here it is….and this table is called….modern periodic table. Let's discuss which law this modern periodic table is based on in detail.
TABLE OF CONTENTS
- Modern Periodic Law
- Periodicity
- Long form of the Modern Periodic Table
- Important Characteristics of the Long Form of Periodic Table
- Periodic Classification of Elements
- Practice Problems
- Frequently Asked Questions - FAQ
Modern Periodic Law
Henry Moseley, an English physicist, in 1913 observed regularities in the characteristic X-ray spectrum of elements. He studied the frequency ()of the X-ray spectra of elements produced by the bombardment of a strong beam of electrons on a metal target. He found that the plot of the frequency (√) of X-rays against the nuclear charge or the atomic number (Z) gave a straight line and not the plot of the frequency of X-rays (√) against atomic mass as thought earlier. He gave the relation
√=a(Z-b)
Where, is the frequency of the emitted X-rays
Z is the nuclear charge or atomic number
a and b are constants.
From his observation, Moseley related the properties of elements with their atomic numbers and gave the new periodic law, known as the ‘Modern Periodic Law’.
"The physical and chemical properties of the elements are periodic functions of their atomic numbers"
- The periodic functions of an element's atomic number, which corresponds to the number of protons or electrons in a neutral atom, determine its physical and chemical properties.
- The periodic variation in electronic configurations, which controls the physical and chemical properties of elements and their compounds, is very naturally what leads to the Modern Periodic Law.
- The 94 naturally occurring elements were shown to have significant similarities by the Periodic Law. Pitch blende, an ore of uranium, also contains neptunium and plutonium, as well as actinium and protactinium. Only in labs or nuclear reactors have elements with atomic numbers from 95 to 118 been synthesised.
Periodicity
- When the elements are placed in the periodic table in the increasing order of atomic numbers, a pattern known as periodicity emerges in their physical and chemical characteristics. It reflects the modern periodic law.
- The melting and boiling points, atomic radii, ionisation energy, electron gain enthalpy, electronegativity, etc. are a few examples of periodic characteristics.
- The periodicity results from the elements' comparable outer electronic configurations occurring at regular intervals.
- Example: Alkali metals, which belong to group 1, have an identical outer electronic configuration, ns1. "n" is the principal quantum number of the outermost shell. The outer electronic structures of group 17 (halogens) elements are also similar, being ns2np5. As a result, they share a lot of characteristics since they have seven valence electrons.
Long form of the Modern Periodic Table
- The elements in the periodic table are arranged in a systematic manner according to their increasing atomic number and recurring chemical properties.
- Vertical columns and horizontal rows are referred to as groups and periods, respectively, in the modern periodic table. They are set out in a tabular format, where a row corresponds to a period and a column to a group.
- The groups and periods of the contemporary periodic table are shown in the following GIF.
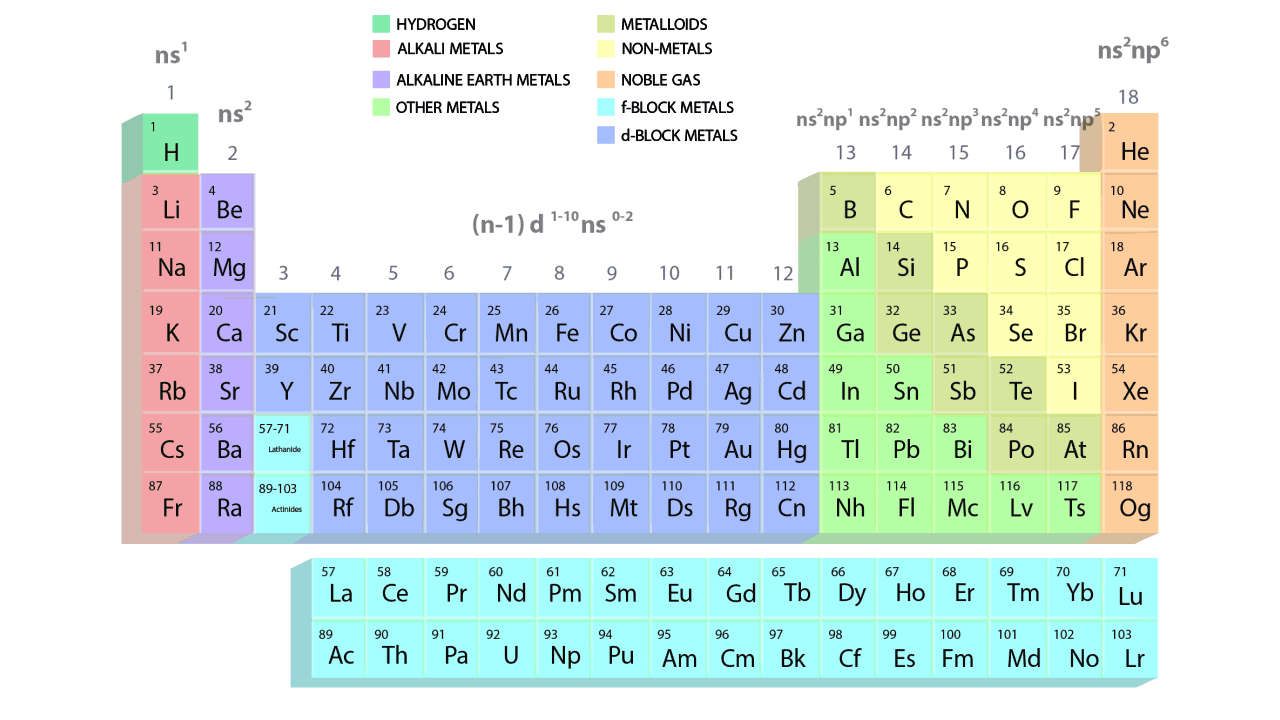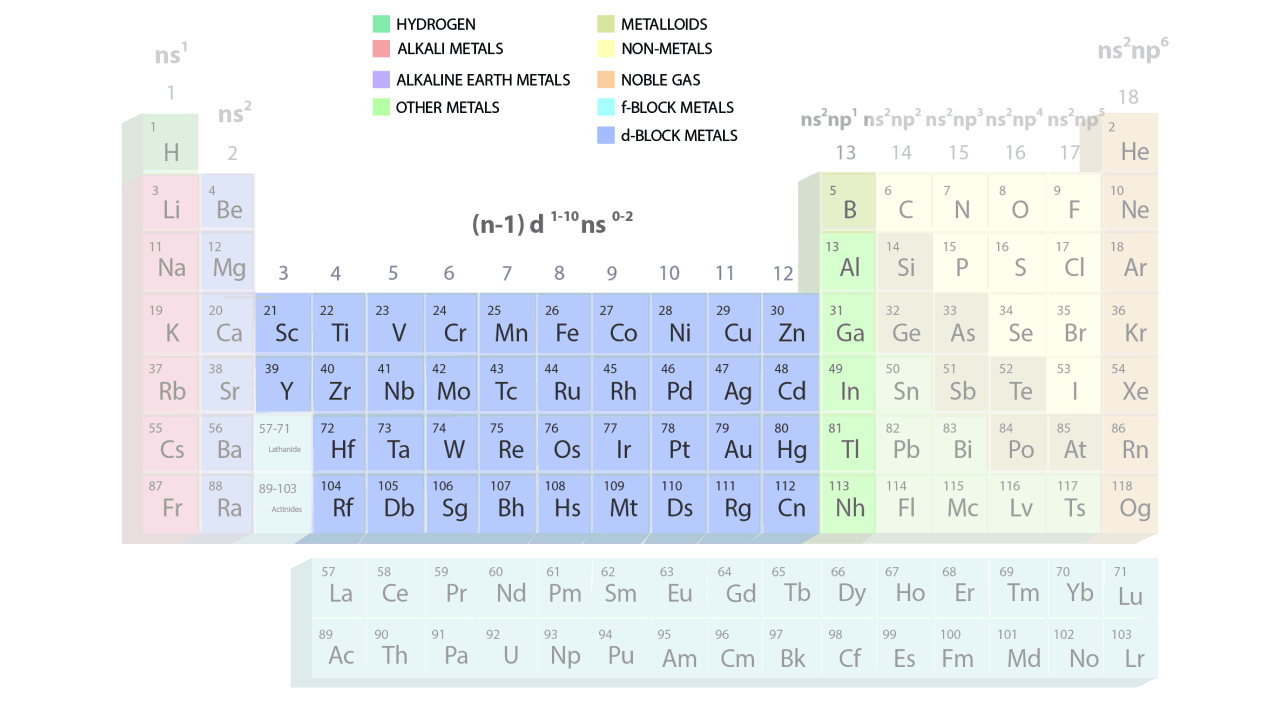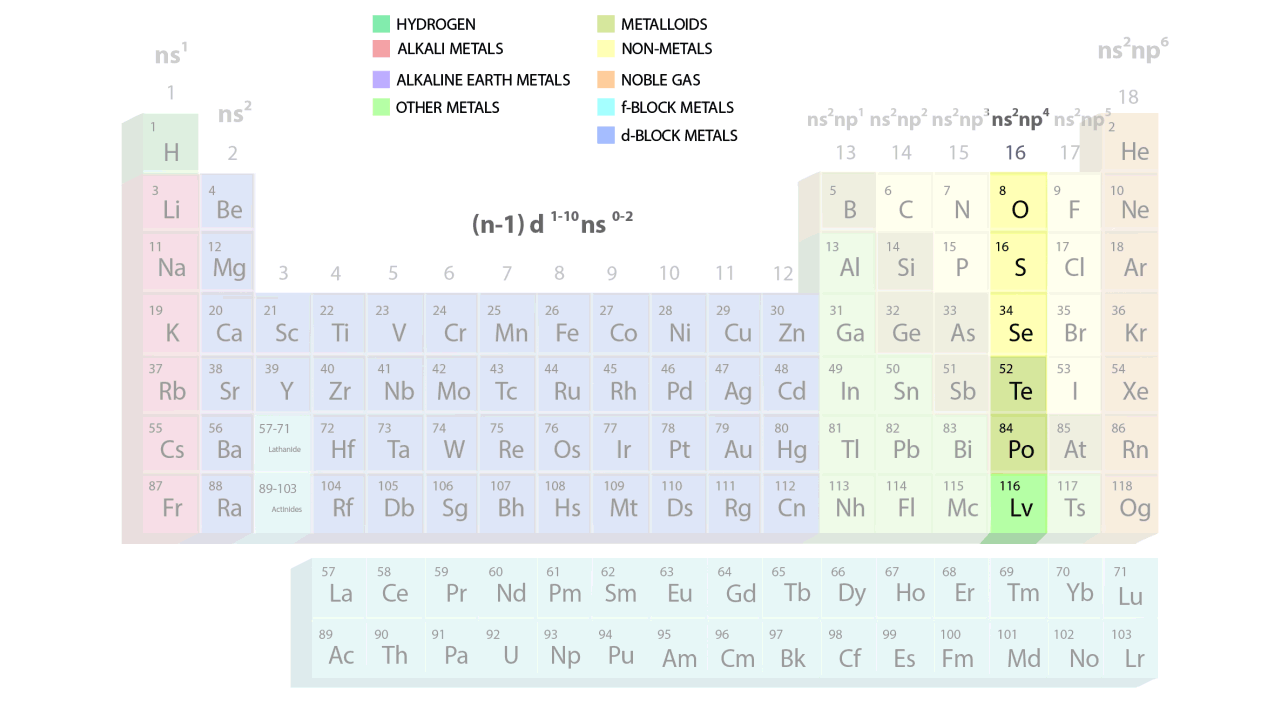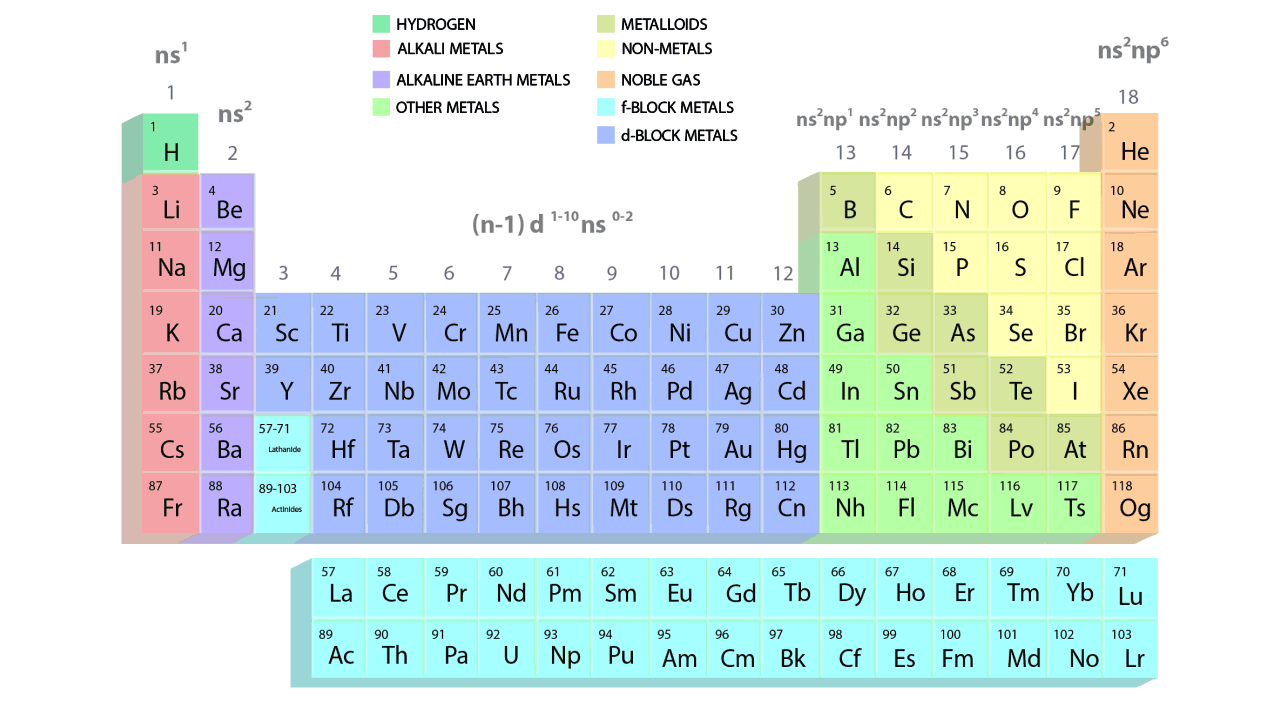
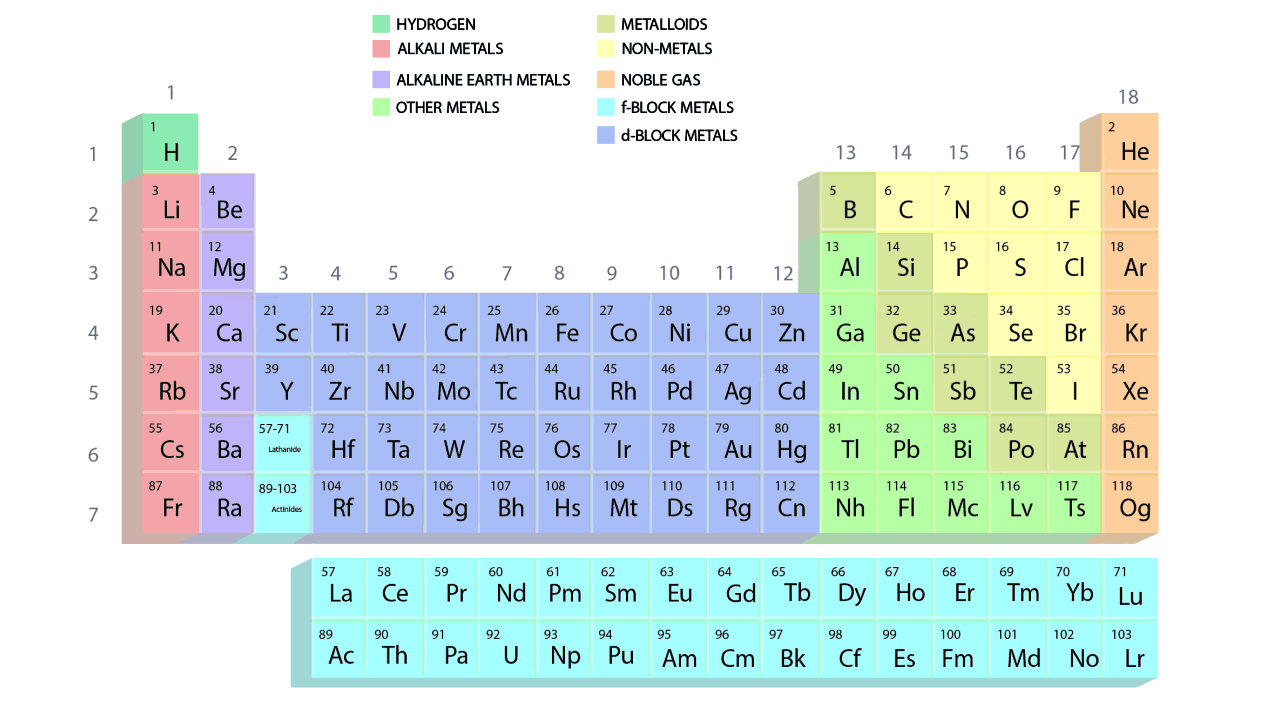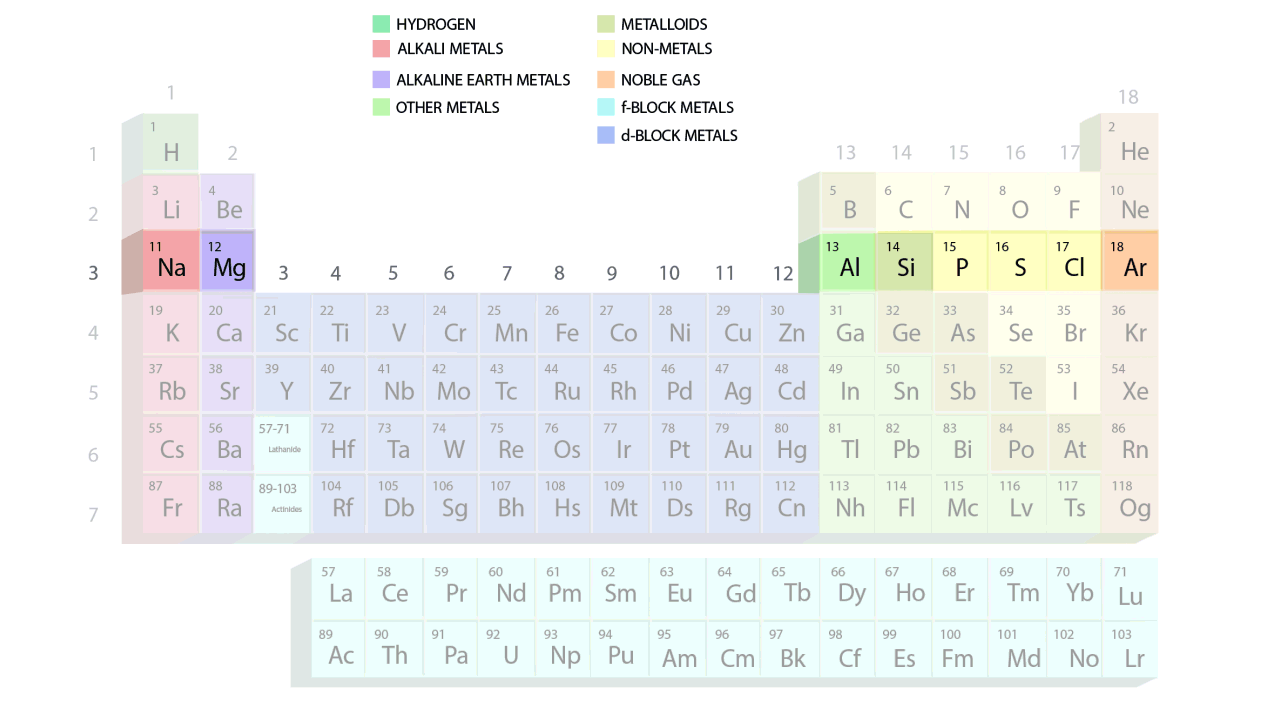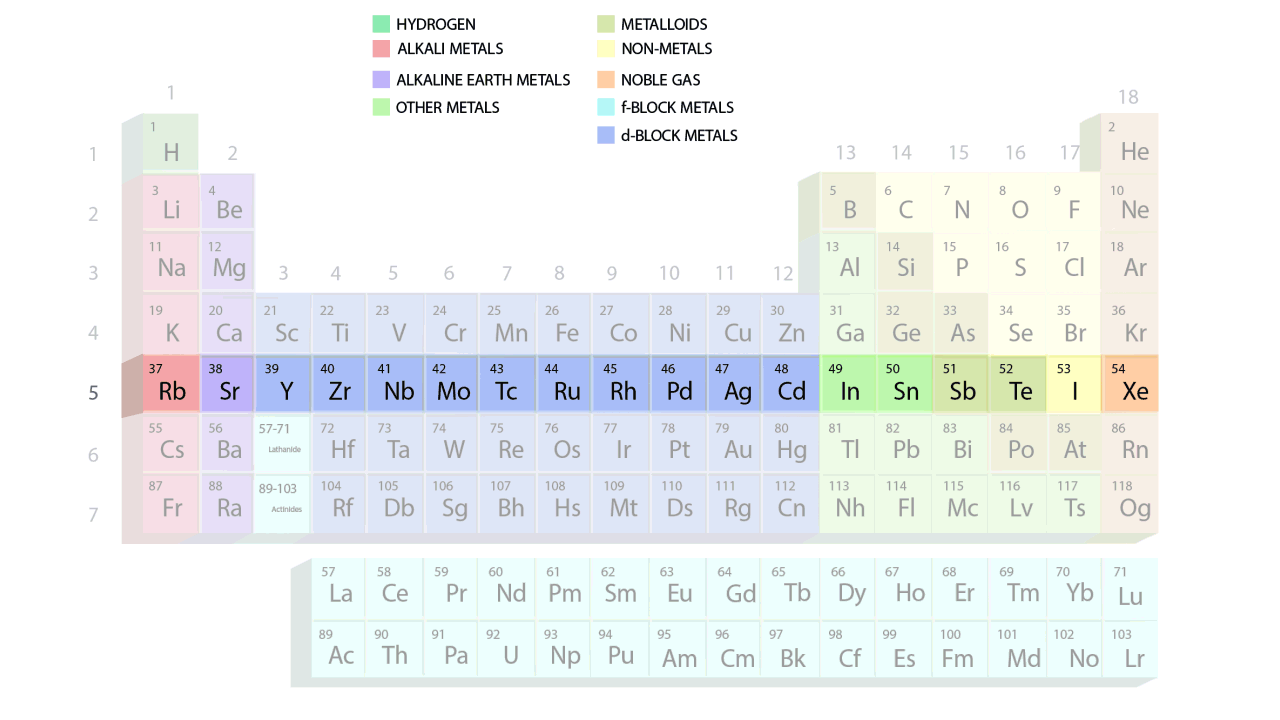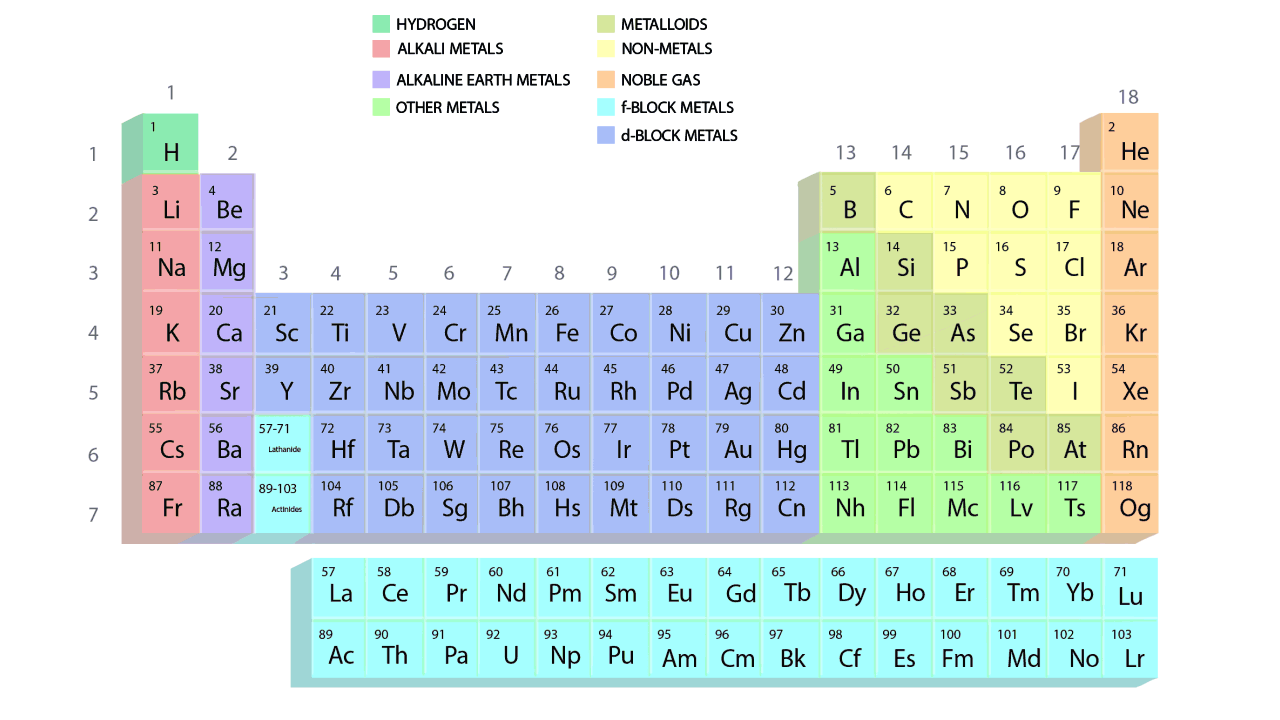
Important Characteristics of the Long Form of Periodic Table
- The modern periodic table has the elements listed in the ascending order of their atomic numbers (Z).
- There are 18 vertical columns, also known as groups, and a total of 7 horizontal rows, also known as periods.
- Elements are grouped in identical vertical columns, known as groups, and have similar outer electronic configurations.
- The period of the element is determined by the principal quantum number, "n." The element in a period with the largest principal quantum number (n) is related to that period.
- One of the four quantum numbers is the principal quantum number (n) (n, l, m, and s). For instance, it shows that the principle shell is 3 if n = 3.
- From left to right, the elements are organised according to increasing atomic numbers.
- The valence electrical configuration of elements in the same group will be the same, and as a result, their chemical characteristics will be identical.
- Valence electrons in the same period, on the other hand, will be ordered in ascending order. Therefore, as the energy level of the atom increases, there are more energy sublevels per energy level.
- The group number of an element is matched by the electrons in the valence shell.
Practice Problems
1. The horizontal rows and vertical columns of the modern long-form of the periodic table are referred to as _________ and ___________, respectively.
- groups, periods
- periods, groups
- rows, columns
- columns, rows
Answer: B
Solution: In the modern periodic table, which is the most practical and generally used long-form periodic chart, the horizontal rows and vertical columns are referred to as periods (series in Mendeleev's terminology) and groups, respectively.
So, option B is the correct answer.
2. The outer shell electronic configuration of argon is
- 3s2 3p5
- 3s2 3p6
- 3s2 3p4
- 3s3 3p5
Answer: B
Solution: Argon belongs to noble gases group and it belong to the this period of the periodic table. Hence, the outer shell electronic configuration of argon is ns2 np6.
So, option B is the correct answer.
3. The period’s number corresponds to the highest _________.
- Azimuthal quantum number
- Spin quantum number
- Magnetic quantum number
- Principal quantum number
Answer: D
Solution: As seen in the most convenient and widely used periodic table of the long-form that is the modern periodic table, the horizontal rows that depict the period number represent the highest principal quantum number of the atoms in the period.
So, option D is the correct answer.
4. Which of the following does not belong to halogen family ?
- I
- Sc
- Br
- Cl
Answer: B
Solution: The six elements in Group 17 of the periodic table make up the halogen family. In the periodic table, group 17 is represented by the elements fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). Hence, scandium (Sc) does not belong to this family.
So, option B is the correct answer.
Frequently Asked Questions - FAQ
1. What are the shortcomings of the modern periodic table?
Answer:
- Uncertainty exists regarding the placement of hydrogen, namely whether it belongs in the IA group or the VIIA group.
- Elements' isotopes have no position in the modern periodic table.
- Actinides and Lanthanides are not included in the Modern Periodic Table and are preserved separately under the table.
- The origin of periodicity, or the separation of related elements, could not be explained by the modern periodic table.
2. Why is the first period known as the shortest period?
Answer: The first period of the periodic table is regarded as the shortest period among the seven periods since it only consists of two elements: hydrogen, which is a member of the alkali metal group, and helium, a noble gas.
3. Which is the incomplete period in the long-form of the modern periodic table?
Answer: The 7th period follows the rule that the elements fill their 7s subshell first and then 5f, 6d and 7p (Plutonium is the exception). As the elements weren’t discovered completely, it is called the incomplete period in the long form of the modern periodic table.
4. How do they finalise on naming a newly discovered element?
Answer: The IUPAC encourages discoverers to suggest names for new elements, which it subsequently takes into consideration and puts up for approval. The IUPAC follows custom for these names and permits the following names to be given to new elements:
1. a legendary idea or figure (including an astronomical object)
2. a mineral or comparable substance
3. Location or geographic area
4. Feature of the Element
5. Scientist
Recommended Videos
Periodic Properties Class 11 Chemistry One Shot | NEET 2022 Chemistry Exam Preparation
20 Most Important Questions from Periodic Properties | Periodic Table NEET Questions | NEET 2022
Periodic Properties Class 11 Chemistry One-Shot (Full Chapter Revision) | JEE Main 2022 Exam Prep


