-
Call Now
1800-102-2727
Kolbe’s Reaction – Mechanism, Examples, Applications, Practice Problems and FAQ
Kolbe's Reaction, also known as the Kolbe-Schmitt Reaction, is a carboxylation reaction. The final product is an aromatic hydroxy acid known as Salicylic Acid.
Carboxylation is the process that occurs when carboxylic acid (R-COOH) is produced by treating a substrate with carbon dioxide (CO2).
The Kolbe reaction is comparable to the Grignard reagents with carbon dioxide reaction in terms of mechanism. Because the phenolate ion has a higher electron density at C-2 or C-4, either carbon atoms can behave as a nucleophile and attack the carbon atom in carbon dioxide.
Hermann Kolbe and Rudolf Schmitt discovered and named this reaction. Hermann Kolbe made significant contributions to the birth of modern organic chemistry.

TABLE OF CONTENTS
- Kolbe’s Reaction
- Mechanism of Kolbe’s Reaction
- Examples of Kolbe’s Reaction
- Applications of Kolbe’s Reaction
- Practice Problems
- Frequently Asked Questions - FAQ
Kolbe’s Reaction
Electrophilic aromatic substitution (SNAr) reactions occur when an electrophile replaces one or more hydrogen atoms on an aromatic ring. Because of their high electron density, phenol and phenoxide ions are highly prone to electrophilic substitution processes.
In phenol, the hydroxyl group linked to the aromatic ring promotes effective charge delocalisation in the aromatic ring. As a result, it stabilises the arenium ion via resonance. The hydroxyl group acts as an ortho-para directing group.
When phenol (C6H5OH) reacts with sodium hydroxide (NaOH), it forms sodium phenoxide (C6H5ONa), due to the abstraction of the acidic-proton. The resulting negative charge is delocalised throughout the benzene ring making the system more electron-rich and thereby more reactive towards electrophilic aromatic substitution reaction. Then, in the presence of H+ (any acid such as sulphuric acid, H2SO4), sodium phenoxide is treated with carbon dioxide (CO2) to produce salicylic acid (2-Hydroxybenzoic acid) and 4- hydroxybenzoic acid.
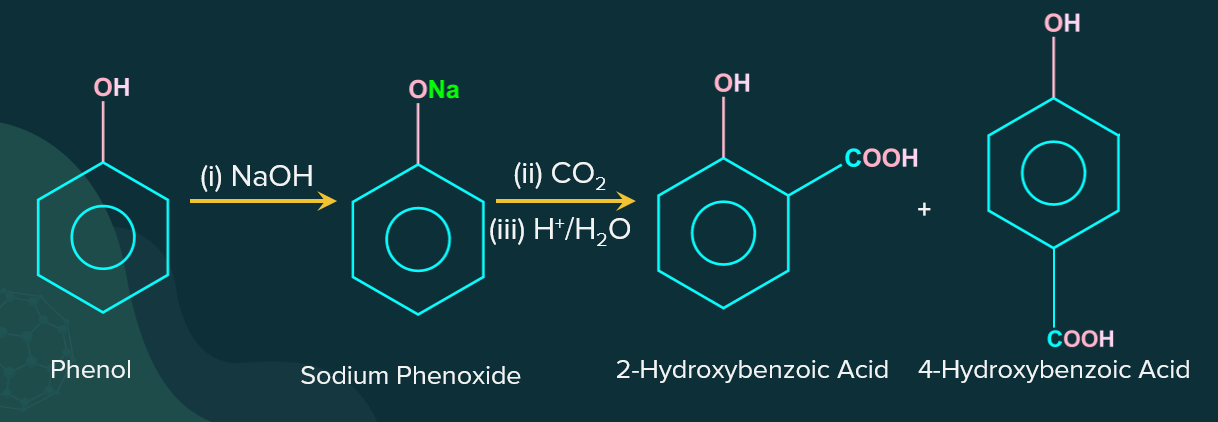
The ortho-isomer is more predominant at lower temperatures, while the para-isomer is obtained at higher temperatures. This is because of the difference in electronegativity of oxygen and the surrounding atoms. All the oxygen atoms in salicylic acid will have a partial negative charge. The oxygen atom, which shares a double bond with the carbonyl carbon in the acid group, is the most electronegative and easily accessible of all. Because of the presence of oxygen, the hydrogen atom in the hydroxyl group will have a partial positive charge. Between these two atoms, hydrogen bonding will occur.

We can see that 2-hydroxybenzoic acid forms intramolecular hydrogen bonds, whereas 4-hydroxybenzoic acid forms hydrogen bonds with other molecules and forms intermolecular hydrogen bonds. When compared to the boiling point of 2-hydroxybenzoic acid, intermolecular hydrogen bonding considerably increases the boiling point of the para-isomer.
Because the boiling point of 4-hydroxybenzoic acid is greater than that of 2-hydroxybenzoic acid, its formation will require more energy. This energy will be provided at higher temperatures, and the reaction will tend to form para-isomers. At lower temperatures, the production of para-isomers is unfavourable, hence ortho-isomers are formed.
|
Property |
o - Hydroxybenzoic acid |
p - Hydroxybenzoic acid |
|
Appearance |
White crystalline |
White crystalline |
|
Odour |
Odourless |
Odourless |
|
Density |
1.44 g cm-3 |
1.46 g cm-3 |
|
Melting point |
434 K |
487 K |
|
pKa |
2.98 |
4.54 |
Mechanism of Kolbe’s Reaction
Step 1: Phenol interacts with sodium hydroxide (OH-) to form phenoxide ion.
The resonating structures of phenoxide ion are as follows.

Step 2: The negative charge on the phenoxide ion shifts to the ortho position and creates a transition state with Na+ and carbon dioxide (CO2) at the same time.
Step 3: The -ve ion attacks the carbon of carbon dioxide (CO2) and the proton shifts from ortho position to oxygen attached to the benzene ring.
Step 4: As a result of the proton shift, H+/ H3O+ (from any mineral acid such as hydrochloric acid, HCl) attacks the carboxylate ion.

The complete mechanism of Kolbe’s reaction can be represented as follows.


Examples of Kolbe’s Reaction
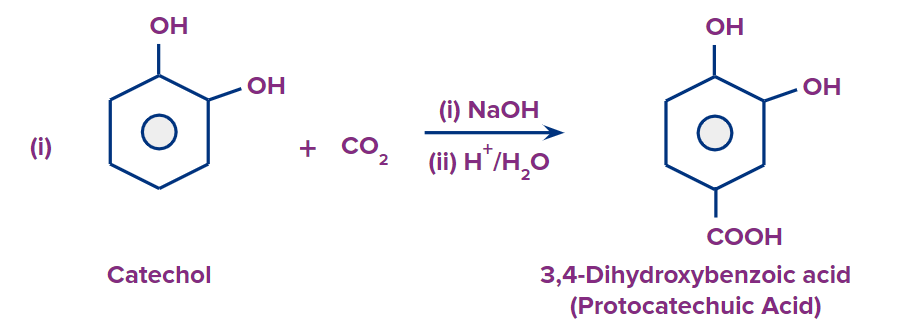
In the presence of a suitable base (like NaOH), dihydroxybenzenes react with carbon dioxide (CO2) to produce dihydroxy carboxylic acids.


Applications of Kolbe’s Reaction
- Salicylic acid on esterification (reacts with acetic anhydride) forms aspirin. In medicine, aspirin is used as a pain reliever.

- Salicylic acid is used to make 3-hydroxy 2-naphthoic acid, which is used to make a variety of dyes and pigments.
- Salicylic acid is also used to produce 4-hydroxybenzoic acid. It is an important chemical utilised in the manufacture of personal care or cosmetic products.
Practice Problems
1. Out of ortho hydroxybenzoic acid and para hydroxybenzoic acid which has a higher melting point?
- ortho hydroxybenzoic acid
- Para hydroxybenzoic acid
- Both have the same melting point
- None of these
Answer: B)
Solution: To melt a compound, the intermolecular forces that hold it together must be broken down. An intramolecular hydrogen bond is established in o-hydroxybenzoic acid. However, this connection does not keep the molecules together. When the molecules are separated, this hydrogen bond will be present within the molecule. So, it makes no difference.
However, in p-hydroxybenzoic acid, intermolecular hydrogen interactions keep the various molecules together, requiring more energy to separate them. Because the hydrogen bonds must be broken, the melting point is higher.
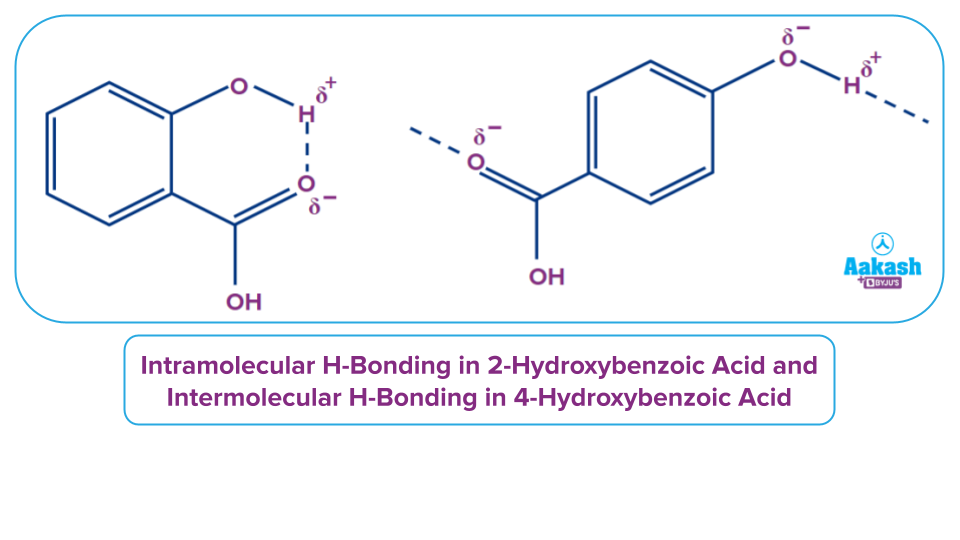
Therefore, the para isomer (215o C) has a higher melting point than the ortho isomer (158o C).
So, option B) is the correct answer.
2. Out of ortho hydroxybenzoic acid and para hydroxybenzoic acid, which is more acidic?
- Ortho hydroxybenzoic acid
- Para hydroxybenzoic acid
- Both
- None of these
Answer: A)
Solution: In ortho-hydroxybenzoic acid, the intramolecular hydrogen bond present in the conjugate base between the COO-and -OH groups produce a six-membered ring. Because this structure is relatively stable, acid deprotonation is promoted, and the compound becomes more acidic.
In the case of the para-isomer, there is substantially less stable hydrogen bonding (intermolecular hydrogen bonding) between distinct molecules. As a result, its acidity is lower than that of the ortho-isomer.
So, option A) is the correct answer.
3. What electrophile is used in the Kolbe reaction?
- Carbon dioxide
- Carbon monoxide
- Boron trifluoride
- Aluminium chloride
Answer: A)
Solution: Carbon dioxide is the electrophile used in Kolbe's reaction. Phenoxide ion is more reactive than phenol in the electrophilic aromatic substitution process. As a weak electrophile, the molecule undergoes a substitution reaction with carbon dioxide. The central carbon atom is bonded to two oxygen atoms in CO2. Because of oxygen's electronegativity, carbon has a partial positive charge, making carbon dioxide an electrophile (Carbon is electron-deficient).
So, option A) is the correct answer.
4. Which is the major product in Kolbe's reaction at low temperatures?
- Ortho hydroxybenzoic acid
- Para hydroxybenzoic acid
- Both
- None of these
Answer: A)
Solution:
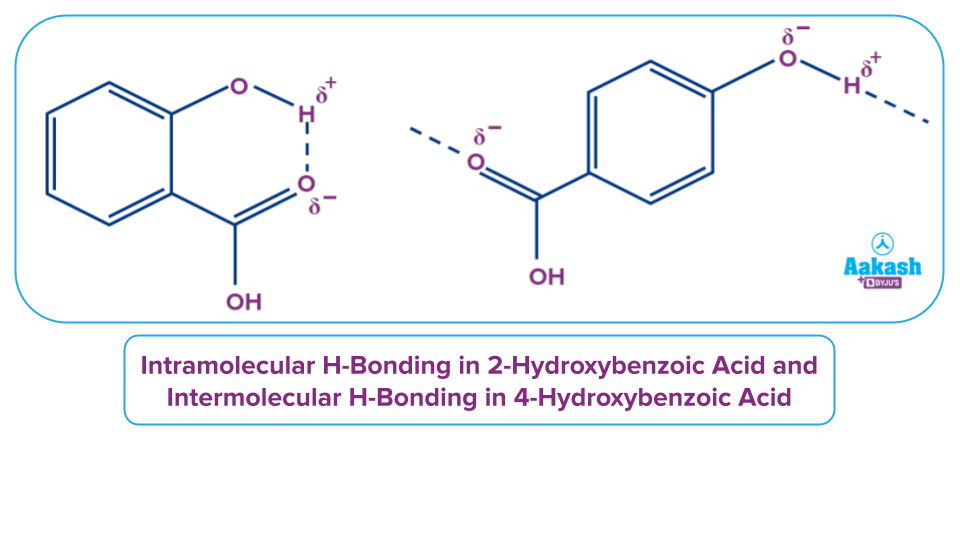
We can see that 2-hydroxybenzoic acid forms intramolecular hydrogen bonds, whereas 4-hydroxybenzoic acid forms hydrogen bonds with other molecules and forms intermolecular hydrogen bonds. When compared to the boiling point of 2-hydroxybenzoic acid, intermolecular hydrogen bonding considerably increases the boiling point of the para-isomer.
Because the boiling point of 4-hydroxybenzoic acid is greater than that of 2-hydroxybenzoic acid, its formation will require more energy. This energy will be provided at higher temperatures, and the reaction will tend to form para-isomers. At lower temperatures, the production of para-isomers is unfavourable, hence ortho-isomers are formed.
So, option A) is the correct answer.
Frequently Asked Questions - FAQ
1. Instead of phenol, phenoxide ion is treated with carbon dioxide in Kolbe's reaction. Why?
Answer: In comparison to phenol, the ability of phenoxide ion to supply a lone pair of electrons to the benzene ring is greater. As a result, the reactivity of phenoxide ion to electrophilic substitution reaction is greater than that of phenols. In Kolbe's reaction, phenoxide ion, being a stronger nucleophile, reacts more easily with carbon dioxide (weak electrophile) than phenols. Kolbe's process is also known as the Kolbe - Schmitt reaction.
2. Are Kolbe's reaction, Koble-Schmitt reaction, and Kolbe's electrolysis all the same?
Answer: Kolbe’s and Kolbe-Schmitt reactions are the same and are examples of carboxylation reactions. Kolbe's electrolysis, on the other hand, is the inverse of Kolbe's reaction, which involves the decarboxylation of carboxylate salts (e.g., potassium carboxylate) into alkanes.
3. Does Kolbe’s electrolysis have any limitations?
Answer: Kolbe's electrolysis does indeed have limitations. These are a few of the notable ones.
1. Alkanes with only even number of carbons can be synthesised by this method
2. This process can't be used to prepare methane.
4. What is Kolbe’s electrolysis method?
Answer: Kolbe's electrolysis method is a general method for preparing substituted hydrocarbons from substituted carboxylic acids using an electric discharge approach that produces carbon dioxide gas. It is one of the most common processes for producing alkanes and substituted hydrocarbons.
The Kolbe reaction is a radical process involving decarboxylative dimerisation. In this reaction, an aqueous solution of sodium or potassium salt of carboxylic acid is electrolyzed, resulting in the salt dissociating into carboxylate ion and sodium or potassium ions. This technique is used to produce symmetrical ethane and higher alkanes.


