-
Call Now
1800-102-2727
Homologous series: Definition of Homologous series, Characteristic of a homologous series, Functional Groups, Practice problems, FAQs:
Which is your favorite chocolate?
We all love chocolates right? Have you ever tried buying chocolate from a supermarket. If you have done so then you know how everything is well organized there. All the chocolates are kept at a certain place so that you can pick and choose easily. So also of other consumables there.

What would happen if everything in supermarket is messed up?
It will be a nightmare for you to find even a single product. That’s why they arrange everything in a set. You will see set of pulses arranged in one corner whereas set of soaps are arranged in different corners.
Similarly in organic chemistry you know that there are millions of organic compounds that exists.a few of which have yet to be found. It is almost impossible to study chemical and physical properties of each and every organic compound. Hence, these compounds are classified into several families or homologous series based on properties shown by the compounds.
Let’s understand how these classifications are done and how it will be help you to understand organic chemistry in a much better way.
Table of content
- Definition of Homologous series
- Characteristic of a homologous series
- Functional Groups
- Practice problems
- Frequently asked questions
Definition of Homologous series
- A homologous series is group or family or structurally comparable organic compounds with a general formula.
- All the members of which include the same functional group. And show a gradation in physical and similarity in chemical properties of any two adjacent members.
- Individual members of such a sequence are known as homologues, and the phenomenon is known as homology.
- Successive homologues differ from each other in the molecular formula by -CH2 only.and so differ by 14 u
Examples:
CH3-CH3 (Ethane), CH3-CH2-CH3 (Propane), and CH3-CH2-CH2-CH3 (Butane).
CH3-CH3 + (-CH2) → CH3-CH2-CH3 + (-CH2) → CH3-CH2-CH2-CH3
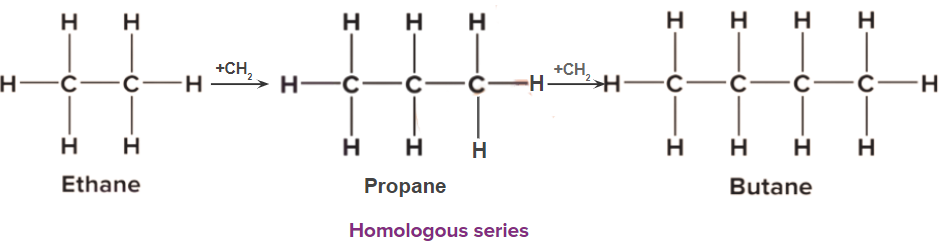
Characteristic of a homologous series
- A general formula can be used to express any homologous series.
- The same functional group is shared by every member of a given homologous series.
All the members of a given homologous series possess the same functional group.
For Example:The hydroxyl group, often known as the -OH group, is the functional group of alcohols.
The aldehydic group, or -CHO group, is the functional group of an aldehyde.
The ketonic group, or -CO group, is the functional group of ketones.
- A -CH2 group distinguishes each consecutive member of a homologous series.
- The general preparatory techniques created for a homologous series can be applied to each individual member.
- The members of homologous series exhibit a predictable gradation in their physical characteristics, such as, melting point, boiling point, and density as the molecular mass increases.
- Although the first member of a homologous series may differ significantly from the other members, they always share comparable chemical characteristics.
Functional Groups
An atom or group of atoms that define the properties and unique chemical reactions of an organic compound is called a functional group.
A functional group is a group of atoms or bonds in the substance that is responsible for the substance’s unique chemical reactions in organic chemistry. The same functional group will behave consistently and undergo similar reactions regardless of the chemical in which it is present.
The general formula and functional groups for many classes of organic compounds, including halides, alcohols, aldehydes, ketones, and organic carboxylic acids, are included in the table below.

Hydrocarbons
- The letter R stands for alkanes, alkenes, and alkynes and occasionally benzene derivatives. Since they exclusively contain carbon and hydrogen atoms, these groups are also known as hydrocarbyl groups. They might differ, though, in the number of double or triple bonds that are between the two carbon atoms.
- The nature of the carbon-carbon bond affects these groups' reactivity in different ways. Some groups have particular names because they contain an alkane that is long and branched or has a ring structure. Names like cyclohexyl and bornyl are examples.
- It's possible for the hydrocarbon functional groups to have an ionic charge. Carbocations are the name given to positively charged compounds, whereas carbanions are the name given to negatively charged hydrocarbons.
Haloalkanes
- The functional groups that have a bond between a carbon atom and a halogen are known as haloalkanes, sometimes known as alkyl halides. "Halo-" is the prefix used to indicate a halogen. For instance, the prefix fluoro can be used to refer to the compound as fluoromethane.
- A halogen is identified by the suffix "halide." For instance, the suffix fluoride can be used to refer to the same substance, fluoromethane , as methyl fluoride.
- Depending on the halogen, the carbon-halogen bond has varying degrees of stability and strength. Alkyl iodides, for instance, have a weak carbon-iodine bond while alkyl fluorides have a strong and stable carbon-fluorine bond.
- All the alkyl halides, with the exception of these alkyl fluorides, are easily subject to elimination reactions or nucleophilic substitution reactions.
Oxygen-containing functional groups
- The hybridization of the carbon-oxygen bond determines all of the features of functional groups that contain this bond.
- This can be explained by the electron donating effect of the sp3 hybridization of oxygen which can be observed in alcohols in sharp contrast with the electron withdrawing effect of the sp2 hybridized oxygen which can be observed in the carbonyl groups which contain a carbon-oxygen double bond.
Practice problems
Q. 1. belongs to which of the following homologous series?
- Alkene
- Alkane
- Alkyne
- alcohol
Answer: (B)
Solution: It is defined as a series of similarly constituted compounds in which the members possess the same functional group, have similar chemical characteristics, and have a regular gradation in their physical properties.
To decide the homologous series of the above compound, we need to find out the general constitution formula of this compound. All the homologous series whether it is alkane, alkane or alkyne are assigned some general molecular formula. If a compound satisfies the general molecular formula then it will be considered as the homologous series of the respective functional group.
Step 1
Look at the number of carbon atom present in the compound.
Here it is 4
Step 2
Divide the number of hydrogen atoms by the number of carbon atoms.(which is 4 in this case)
Step 3
Check for the remainder and add it with the multiple
Hence,
C4H10 belongs to alkane.
Q.2. What is the mass difference between two consecutive members of an alkene group?
- 34
- 36
- 14
- 12
Answer: (C)
Solution:
An alkene's standard chemical formula is CnH2n.
Where n= 2,3,4,5,6.........
Putting n=2
We get
C2H22=C2H4
Molecular mass of C2H4
Atomic mass of carbon atom =12 amu
Atomic mass of hydrogen atom =1 amu
2Atomic mass of carbon atom+4Atomic mass of hydrogen atom
212+41=24+4=28
Now
Putting n=3
We get
Molecular mass of
Atomic mass of carbon atom =12 amu
Atomic mass of hydrogen atom =1 amu
3Atomic mass of carbon atom+6Atomic mass of hydrogen atom
312+61=36+6=42
Difference of molecular masses between these two compounds is 42-28=14 amu.
Q.3. belongs to which homologous series?
a. Alkane
b. Alkene
c. Alkyne
d. aldehyde
Answer: (C)
Solution:
It is defined as a series of similarly constituted compounds in which the members possess the same functional group, have similar chemical characteristics, and have a regular gradation in their physical properties.
To decide the homologous series of the above compound, we need to find out the general constitution formula of this compound. All the homologous series whether it is alkane, alkane or alkyne are assigned some general molecular formula. If a compound satisfies the general molecular formula then it will be considered as the homologous series of the respective functional group.
Step 1
Look at the number of carbon atom present in the compound.
Here it is 6
Step 2
Divide the number of hydrogen atoms by the number of carbon atoms.(which is 4 in this case)
Step 3
Check for the remainder and add/substract accordingly it with the multiple
Hence,
belongs to alkyne.
Q.4. two successive homologous series differ by
a.
b.
c.
d.
Answer: (A)
Solution:
A homologous series is a series of organic compounds, each of which contains a distinctive functional group.
Successive homologues differ from each other in the molecular formula by -CH2 only.
Examples:
(Butane).
Q.5. Which of the following belongs to an alternative homologous series?
a.
b.
c.
d.
Answer: (C)
Solution:
all belongs to same homologous series.
All have the general formula of CnH2n+2 which is for alkane. Whereas C7H14is an alkex`ne because it satisfies the general formula of alkene which is CnH2n.
Frequently asked questions
Q.1. Do all the homologous series posses similar physical properties?
Answer: Because they share a functional group, homologous series have comparable chemical characteristics. However, due to stronger London dispersion forces, homologues' physical characteristics are different. In addition to organic molecules, other substances can also have homologous series.
Q.2. Why is it necessary to group compounds into homologous series?
Answer: A homologous series of compounds often share comparable chemical and physical properties because they have a fixed set of functional groups. Hence, it becomes easier to predict the property of an organic compound by determining its homologous series. Otherwise it would have been too difficult for us to individual study all the chemical and physical properties of every organic compound. As you know there are millions of organic compounds that exist.
Q.3. Is there any homologous series for inorganic compounds?
Answer: The phosphoric acids, silicic acids, and phosphonitrilic chlorides are homologous series of inorganic chemicals. The chemical properties of the compounds are basically identical within a given homologous series, whereas the physical qualities fluctuate continuously and predictably.
Q.4. Are paraffines and alkanes same thing?
Answer: Alkanes are known as paraffins because of their little affinity for a common reagent. Alkanes are therefore inactive compounds. Under harsh circumstances, they experience reactions.


