-
Call Now
1800-102-2727
Half Reaction Method - Redox Reaction, Half Reaction Method of Balancing Equations, Practice Problems and FAQs
We had to have gone to the fruit and vegetable market together. But have you ever noticed the weighing scale used by traditional vendors to measure fruits and vegetables?
You may recall from the image that a traditional scale consists of two plates or bowls held in place by a lever at intermediate points.
Unknown masses are placed on one plate, while known masses are placed on the other until the masses on both plates are equal.
This is precisely what we are doing when balancing chemical equations. We split the reaction into two halves, and balance it with respect to each other to get the overall balanced equation.
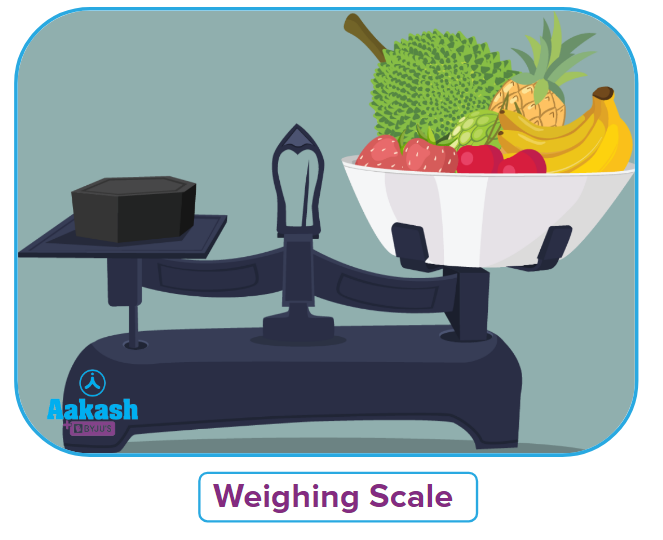
Table of Contents
- Redox Reactions
- Half Reaction Method of Balancing Equations
- Practice Problems
- Frequently Asked Questions
Redox Reactions
In reactions involving ions, some ions undergo valency or changes by either accepting or giving away electrons. Redox reaction is a chemical reaction in which electrons are exchanged between two distinct reactants. As a result, they undergo changes in oxidation number.
The substance or more correctly atom or ion that gives away electrons gets a higher positive charge and is said to be oxidized. An atom or ion shows an increase in oxidation number. The process involving this giving away of the electron is called reduction reaction or process.
Similarly, the atom or ion that gains electrons gets a higher negative charge and is said to be reduced. The atom or ion shows a decrease in oxidation number. The process of accepting electrons is called oxidation.
Both reactions are dependent on each other and hence take place simultaneously. It is feasible to determine the transfer of electrons between the relevant reactants by looking at the change in oxidation status of both reacting species.
A reduction-oxidation or redox reaction is a chemical process in which both reduction and oxidation take place at the same time. While the reduced species gets electrons, the oxidised species loses electrons.
Any chemical reaction has to be stoichiometrically balanced in terms of mass and elements.
Redox reaction equations can be balanced by two methods-
- Oxidation number method and
- Half reaction or ion-electron method.
Half Reaction Method of Balancing Equations
This method is also known as the Ion-Electron Method. This approach to reaction balancing is based on the idea that electrons lost during any redox reaction's oxidation half-reaction are equivalent to electrons gained during that reaction's reduction half-reaction.
Hence, the whole reaction is separated into two half-reactions of reduction and oxidation. These two halves' reactions are separately balanced for electrons and summed up to get the stochiometric equation.
The following steps are used to balance a chemical equation using the ion-electron method or Half Reaction method:
Step 1: Leave the spectator or nonparticipating species.
Identify the oxidation number of species that undergo changes
Step 2: Divide the entire equation into two half-reactions of oxidation and reduction with the corresponding reactant and products.
Step 3: Balance half equations, individually by electrons to compensate for the oxidation number change, with water for oxygen difference and with H+ or OH- for acid or base medium.
Step 4: Multiply one or both half equations by appropriate numbers so that when the two equations are added, the electrons are balanced.
Let us consider an example of a reaction in an acidic medium that makes the method more understandable In this reaction, potassium permanganate reacts with sodium sulfite to form sodium sulfate and manganese ion. Sodium and potassium do not take part in the reaction and are spectator ions and can be ignored.
Step 1: Identify the elements whose oxidation numbers have been altered. Select the substance that is oxidised and reduced which helps to identify the oxidation-reduction reaction.
The oxidation state of Mn in = x
Oxidation state of Mn in Mn2+ = y
y= +2
Oxidation state of Mn changes from +7 to +2, Hence get reduced by taking 5 electrons.
Oxidation state of S in = a
x= +4
Oxidation state of S in SO42- = b
b+4(-2)=-2
x= +6
Oxidation state of S changes from +4 to +6, Hence get oxidised by giving away 2 electrons.
Step 2: Divide the entire equation into two half-reactions, one for the oxidation and one for the reduction as per the prior step.
Reduction:
Oxidation:
Step 3: Balance half equations using the steps below:
(i) Balance every atom except H and 0.
They are already balanced as they contain one Mn and one S on both side
Reduction:
Oxidation:
(ii) on both sides of the equation, compute the oxidation number. To make up the difference, add electrons to whichever side is required.
Reduction:
Oxidation:
(iii) balance the half equation so that both sides receive the same charge.
- If the medium is acidic, balance the charge by adding H+ ions.
- If the medium is basic, balance the charge by adding OH- ions.
As the reaction is in an acidic medium
Reduction:
Oxidation:
(iv) Complete the balancing of the equation by adding water molecules.
Reduction:
Oxidation:
Step 4: Multiply one or both half equations by appropriate numbers so that when the two equations are added, the electrons are balanced.
Reduction:
Oxidation:
Reduction: 2
Oxidation: 5
Step 5: Combine two balanced half-equations.
Overall reaction: 2
It should be noted that redox reactions can occur in any of the three media, namely acidic, basic, or neutral.
Practice Problems
Q1. Balance the given reaction equation in the basic medium and select the accurate reduction half-reaction for the same reaction equation
Answer: (D)
Solution:
Step 1: Identify the elements whose oxidation numbers have been altered. Select the substance that is oxidised and reduced which helps to identify the oxidation-reduction reaction.
Oxidation state of Br in Br2 = x
2x=0
x= 0
Oxidation state of Br in = y
y+3(-2)= -1
y=+5
Oxidation state of Br changes from 0 to +5, Hence Br2 get oxidised .
Oxidation state of O in H2O2 = a
2+2a=0
a=-1
Oxidation state of O in H2O = b
2+b=0
b=-2
Oxidation state of O changes from -1 to -2, Hence H2O2 get reduced.
Step 2: Divide the entire equation into two half-reactions
Oxidation:
Reduction:
Step 3: Balance half equations using the steps below:
(i) Balance every atom except H and 0.
Oxidation:
Reduction:
(ii) Add electrons to whichever side is required.
Oxidation: (each Br loses 5 e-)
Reduction: (each O loses 1 e-)
(iii) As the reaction is in basic medium, the balance the charge by adding OH- ions.
Reduction:
Oxidation:
(iv) Complete the balancing of the equation by adding water molecules.
Oxidation:
Reduction:
:
Hence the correct answer is option (D).
Q2. Balance the given reaction equation in an acidic medium and select the accurate oxidation half-reaction for the same reaction equation
Answer: (B)
Solution:
Step 1: Identify the elements whose oxidation numbers have been altered. Select the substance that is oxidised and reduced which helps to identify the oxidation-reduction reaction.
The oxidation state of Mn in = x
x+4(-2)=-1
x= +7
Oxidation state of Mn in MnO2 = y
y+2(-2)= 0
y=+4
Oxidation state of Mn changes from +7 to +4, Hence get reduced.
Oxidation state of I in I- = a
a = -1
Oxidation state of I in I2 = b
2b=0
b=0
Oxidation state of I changes from -1 to 0, Hence I- get oxidised.
Step 2: Divide the entire equation into two half-reactions
Reduction:
Oxidation:
Step 3: Balance half equations using the steps below:
(i) Balance every atom except H and 0.
Reduction:
Oxidation:
(ii) Add electrons to whichever side is required.
Reduction:
Oxidation:
(iii) As the reaction is in acidic medium, the balance the charge by adding H+ ions.
Reduction:
Oxidation:
(iv) Complete the balancing of the equation by adding water molecules.
Reduction:
Oxidation:
Hence the correct answer is option (B).
Q3. How many electrons are required to balance the half oxidation and reduction reaction in the given reaction equation in the acidic medium?
- 3, 1
- 6, 1
- 6, 2
- 3, 2
Answer: (C)
Solution:
Step 1: Identify the elements whose oxidation numbers have been altered. Select the substance that is oxidised and reduced which helps to identify the oxidation-reduction reaction.
The oxidation state of Cr in = x
x= +6
Oxidation state of Cr in = y
y=+3
Oxidation state of Cr changes from +6 to +3, Hence get reduced.
Oxidation state of C in C2H4O = a
a=-1
Oxidation state of C in C2H4O2 = b
b = 0
Oxidation state of C changes from -1 to 0, Hence C2H4O get oxidised.
Step 2: Divide the entire equation into two half-reactions
Reduction:
Oxidation:
Step 3: Balance half equations using the steps below:
(i) Balance every atom except H and 0.
Reduction:
Oxidation:
(ii) Add electrons to whichever side is required.
Reduction:
Oxidation:
Hence the correct answer is option (C).
Q4. What should be the coefficient of in the given balanced reaction equation in acidic medium?
- 1
- 2
- 6
- 4
Answer: (A)
Solution:
Step 1: Identify the elements whose oxidation numbers have been altered. Select the substance that is oxidised and reduced which helps to identify the oxidation-reduction reaction.
The oxidation state of Zn = x
x= 0
Oxidation state of Zn in Zn2+ = y
y=+2
Oxidation state of Zn changes from 0 to +2, Hence Zn get oxidised.
Oxidation state of N in = a
a=+5
Oxidation state of N in = b
b+4=1
b=-3
Oxidation state of C changes from +5 to -3, Hence get reduced.
Step 2: Divide the entire equation into two half-reactions
Oxidation:
Reduction:
Step 3: Add electrons and H+ ions to whichever side is required.
Oxidation:
Reduction:
(iv) Complete the balancing of the equation by adding water molecules.
Oxidation:
Reduction:
Step 4: Combine two balanced half-equations.
Oxidation:
Reduction:
Oxidation:
Reduction:
Overall reaction:
Coefficient of nitrate ion is one. Hence the correct answer is option (A).
Frequently Asked Questions
Q1. What is Reduction?
Answer: Reduction is the name given to any chemical reaction in which a chemical species gains electrons. Actually, it indicates that the atom or ion had been reduced if its oxidation number decreased.
Q2.What is Oxidation?
Answer: Oxidation is the name given to any chemical reaction in which a chemical species loses electrons. In actuality, it denotes the oxidation of the atom or ion whose oxidation number has increased.
Q3. What is Oxidizing agent / Oxidant?
Answer: An oxidant is a substance that, during a chemical reaction, can both oxidise other substances and reduce itself, or a reagent that obtains electrons during a redox reaction.
Q4. What are Reducing agents / Reductants?
Answer: A reductant is a chemical compound that can reduce others while oxidising itself or a reagent that loses electrons in a redox reaction.


