-
Call Now
1800-102-2727
Freundlich Adsorption Isotherm: Adsorption Isotherm, Mathematical and Graphical Representations of Freundlich Adsorption Isotherm, Practice Problems and FAQs
Can you tell me what is the common factor among the manufacture of most important substances like fertilizers, sulphuric acid and Vanaspati? Yes all of them involve a catalytic process of manufacture. Without catalyst, we would not have been able to feed the world population, nor prepare the king of chemicals,namely sulphuric acid. These catalysts function by adsorption of gaseous reactants and help them to react with other reagents to give the products. The efficiency of the production and catalyst depends much on the extent of adsorption of gasses on the solid catalyst surface.
There are many other small to large applications of this solid -gaseous interaction. So, then knowing the fundamentals of interactions and the amount of interactions becomes all the more necessary. Come let us learn about them.
Adsorption isotherms have been used extensively in research on environmental protection and adsorption strategies. Two of the most common approaches for forecasting a material's adsorption capacity are the Freundlich and Langmuir isotherms.
Adsorption is of two types: physisorption and chemisorption. Because the mechanisms of both types of adsorption differ, we require different isotherms to describe them. This concept page will go into detail
about the Langmuir Adsorption isotherm and the type of adsorption it deals with.
TABLE OF CONTENTS
- Freundlich Adsorption Isotherm
- Mathematical Representation
- Graphical Representation
- Drawback of Freundlich Isotherm
- Practice Problems
- Frequently Asked Questions
Freundlich Adsorption Isotherm
Adsorption is a surface phenomenon, where the adsorbate (molecules that are adsorbed) gets attached to the surface of the adsorbent (substance that attracts the adsorbate). The adsorption can involve physical or chemical forces and accordingly referred as Physisorption and chemisorption respectively.
Physisorption involves multilayer formation on the surface of the adsorbent while chemisorption is always an unimolecular layer adsorption.
The extent of adsorption of these two cases differ much in terms of the nature of forces involved, energy released, effect of temperature etc.
The amount of adsorbate adsorbed is mathematically given by two relations, namely Langmuir and Freundlich adsorption isotherm.
Freundlich gave an empirical relationship between the extent of adsorption(x/m) and pressure(p), at a particular temperature.
Mathematical Representation
The Freundlich relationship between the amount of gaseous adsorbate per unit weight of the adsorbent with respect to the change in pressure is given as,
Where,
k and n: Constants
x: Mass of gas adsorbed
m: Mass of adsorbent
n > 1
Thus, factor (1n) can have values between 0 to 1.
Probable range of n is 0.1 to 0.5.
Thus, Freundlich isotherm holds good over a limited range of pressure and for chemisorption forming a monolayer on surface..
Note: At a particular temperature k and n depends on the nature of the gas and adsorbent.
Taking log on both sides of equation (i)
Graphical Representation
Plot of vs pressure(p) at different temperatures is shown here as-

Lower the temperature higher will be adsorption.
Graphical representation of equation (ii)
p…..(ii)
On comparing equation (ii) with the equation of a straight line, y=mx +c
Slope
Intercept (c) = log k
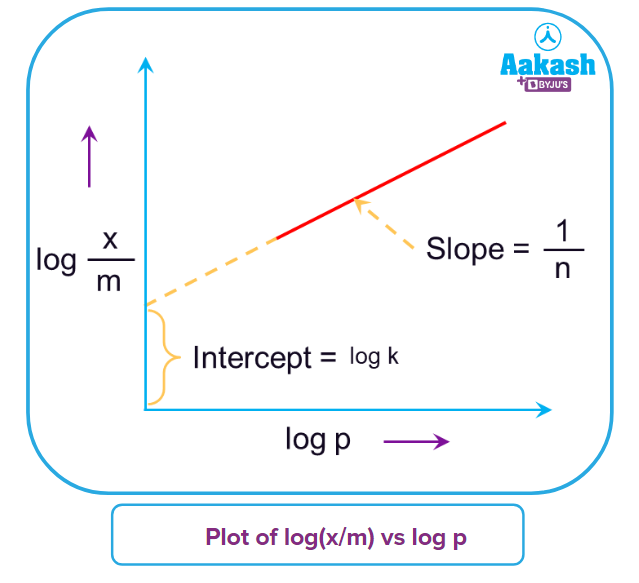
At a fixed pressure there is a decrease in physical adsorption with an increase in temperature.
The curves at different temperatures approach saturation at high pressure.
Drawback of Freundlich Isotherm
One of the major drawbacks of Freundlich isotherm is that it fails to explain the extent of adsorption at high pressure of the gas. Equation (1) is applicable only when an adsorbate substance forms a unimolecular layer on an adsorbent surface. i.e. chemical adsorption.
Practice Problems
1. The value of ‘n’ in Freubdlich’s absorption isotherm ranges between:
(A). 0 to 1
(B). 0.1 to 0.5
(C). 0 to 2
(D). 0.5 to 0.1
Answer: B
Solution: The factor (1n) can have values between 0 to 1.
The probable range of n is 0.1 to 0.5.
2. The plot of log xm vs log p is:
- Straight line plot
- Exponential plot
- Hyperbolic plot
- Gaussian plot
Answer: A
Solution: p…..(ii)
On comparing the above equation with the equation of a straight line, y=mx +c
Slope
Intercept (c) = log k
3. Freundlich's adsorption isotherm represents the adsorption of a gas. In the given plot, x is the mass of the gas adsorbed on the mass m of the adsorbent at pressure P. x/m is proportional to
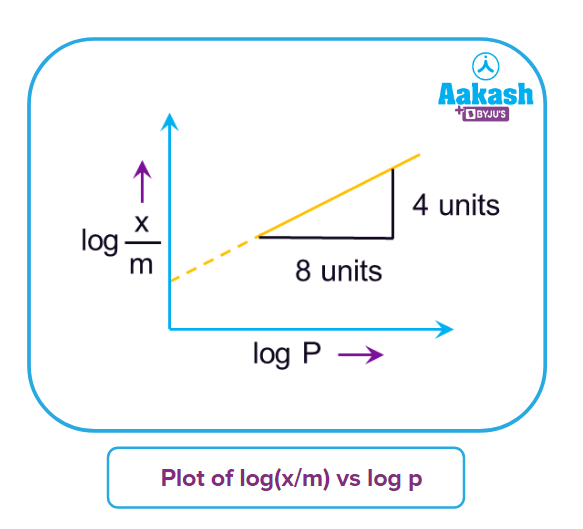
(A) p2
(B)
(C) p
(D) p4
Answer: B
Solution:
Slope
n = 2
4. At a particular temperature k and n depends on the:
(A) Nature of the gas
(B) Nature of the adsorbent
(C) Both A and B
(D) None of the above
Answer: C
Solution: At a particular temperature k and n depends on the nature of the gas and adsorbent.
Frequently Asked Questions - FAQ
Q1. How is Langmuir isotherm different from Freundlich isotherm?
|
Langmuir isotherm |
Freundlich isotherm |
|
|
|
|
Q2. What is adsorption isotherm in general?
The variation in the amount of gas adsorbed by the adsorbent with pressure at constant temperature can be expressed by a curve termed an adsorption isotherm.
Q3. What is adsorption?
It is the accumulation of a molecular species at the surface rather than in the bulk of a solid or a liquid. The attachment of a gas, liquid, or dissolved material to a layer of atoms, ions, or molecules is known as adsorption. The process occurs on the surface of an adsorbent.
Q4. What is the purpose of isotherms?
An isotherm is a line drawn at the same temperature that connects points on a map or graph. In meteorology, isotherms are widely used to observe temperature distribution over the Earth's surface or on a chart indicating steady or constant pressure.


