-
Call Now
1800-102-2727
Factors Affecting SN1 and SN2: Nature of the Substrate, Nucleophile, Solvent, Leaving Group Ability, Difference Between SN1 and SN2
Let us consider a small story of the cats and the comfortable chair to understand this topic better. Here, the grey cat (nucleophile) wants to sit on the chair where the brown cat (leaving group) is already sitting.
So, there are two ways in which a grey cat (nucleophile) sits on the chair. First is the grey cat (nucleophile) waits for the brown cat (leaving group) to leave the chair and once it leaves the seat gets vacant and the grey cat (nucleophile) can easily sit on the chair.

The second way is that the brown cat (nucleophile) occupies the seat simultaneously as the grey cat (leaving group) leaves it. So how to determine in which way will the brown cat occupies the seat?
Is it the mood of the brown cat or grey cat, the environment of the two cats, or some other factors?

Considering this as an analogy for SN1 and SN2 mechanism. What are the factors that determine which out of two mechanisms will be followed?
TABLE OF CONTENTS
- Nature of the substrate
- Nature of the nucleophile
- Nature of the solvent
- Leaving group ability
- Difference between SN1 and SN2
- Practice Problems
- Frequently Asked Questions - FAQs
Nature of the Substrate
Effect of Nature of the Substrate in SN1 reaction:
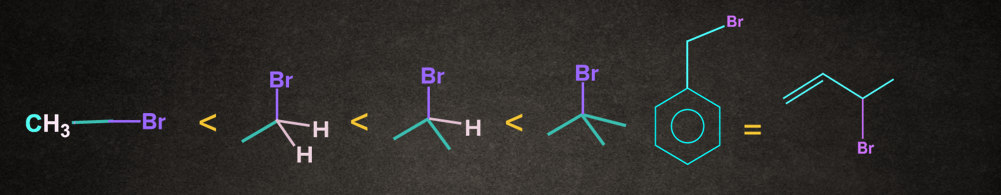
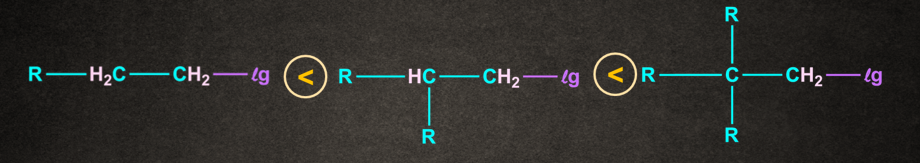
Rate of SN1 Reaction depends on the stability of carbocation. The nature of alkyl halide decides the reactivity rate of SN1 reaction and the stability of the carbocation. Increasing order of reactivity of alkyl halides in the SN1 reaction is:
CH3– X <1o Alkyl halide <2o Alkyl halide < 3o Alkyl halide <Benzylic halide =Allylic halide
|
Alkyl bromide |
Class of alkyl bromide |
Relative rate |
|
|
Tertiary |
12,00,000 |
|
|
Secondary |
11.6 |
|
|
Primary |
≈ 0 |
Also as - branching increases the rate of SN1 reaction increases.
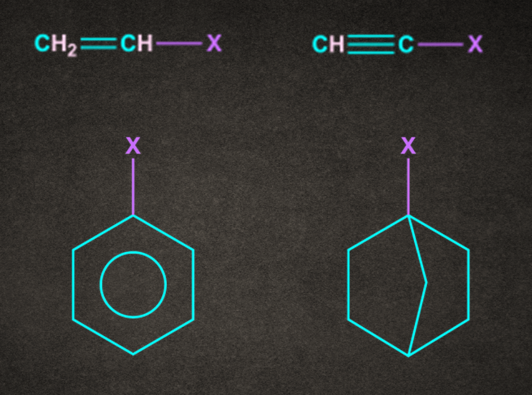
Note: Following compounds generally do not give SN1 reaction because of the formation of an unstable carbocation.
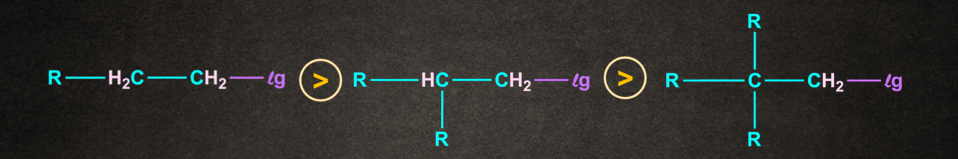
Effect of Nature of the Substrate in SN2 reaction: The important reason behind the order of reactivity in SN2 reaction is the steric effect. Very large and bulky groups can often hinder the formation of the required transition state. The crowding raises the energy of the transition state and slows down the rate of reaction.
Decreasing order of reactivity of alkyl halides is
Benzylic halide =Allylic halide >CH3–X >1o Alkyl halide >2o Alkyl halide > 3o Alkyl halide
|
Substituent |
Compound |
Relative rate |
|
Methyl |
CH3X |
30 |
|
10 R–X |
CH3CH2X |
1 |
|
20 R–X |
(CH3)2CHX |
0.02 |
|
30 R–X |
(CH3)3CX |
∽0 |
Also as - branching increases, the rate of SN2 reaction increases.
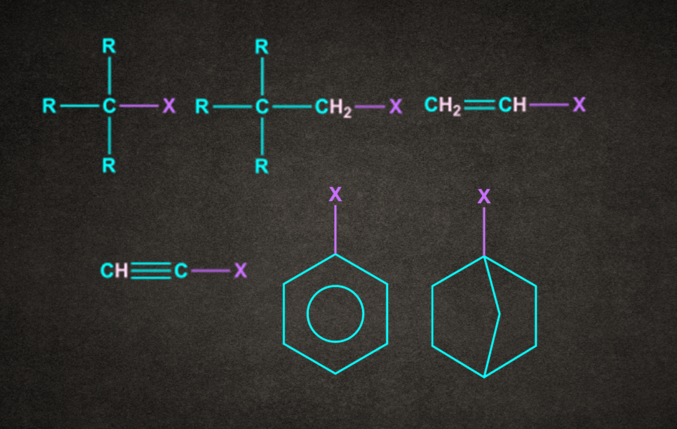
Note: Following compounds generally do not give SN2 reaction.
Nature of the nucleophile
Effect of Nature of the nucleophile in SN1 reaction: The rate of SN1 reaction is unaffected
by the concentration of the nucleophile.
Weak and neutral nucleophiles favors SN1 reaction. Example - H2O, NH3, etc.
Note: Mostly, solvents (protic) function as nucleophiles themselves in a SN1 reaction. So, an SN1 reaction is termed a solvolysis reaction.
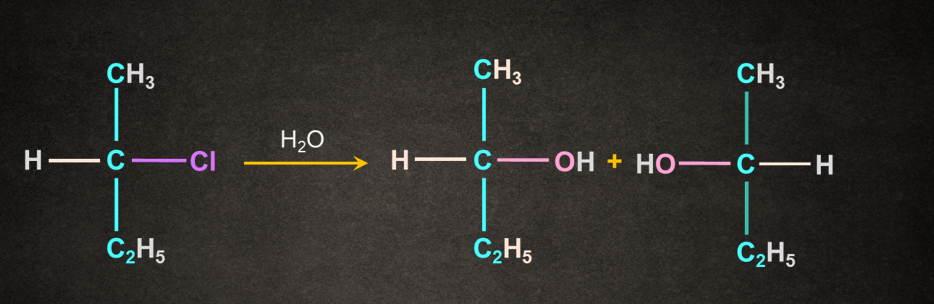
In this case water acts as both solvent and nucleophile.
Effect of Nature of the nucleophile in SN2 reaction: The rate of SN2 reaction depends on the concentration of both alkyl halide and the nucleophile. As the concentration of nucleophiles increases, the rate of SN2 reaction also increases. Also better the nucleophile faster is the rate of reaction. Therefore, anionic nucleophiles mostly give SN2 reaction. Example- OH-, NH2-, CN-, etc.
Nature of the solvent
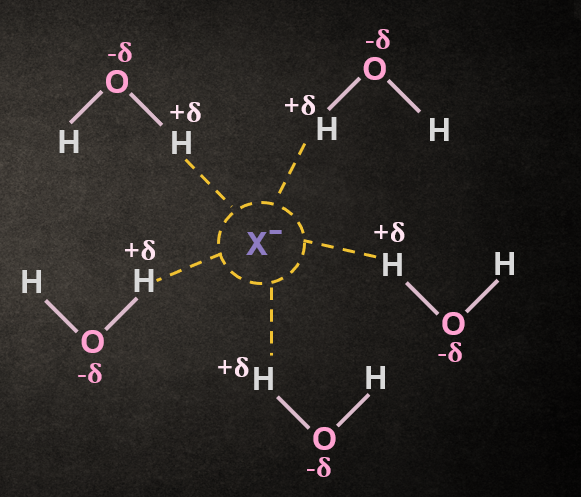
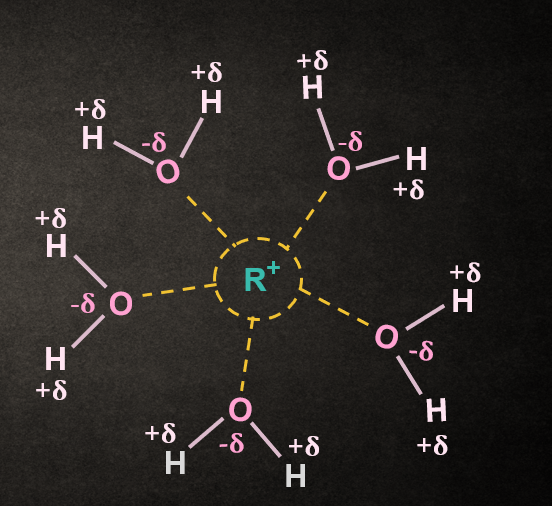
Effect of Nature of the Solvent in SN1 reaction:
The use of a polar protic solvent will greatly increase the rate of ionization of an alkyl halide in any SN1
reaction because it solvate cations and anions effectively and stabilizes the transition state, leading
to the formation of intermediate carbocation and halide ion; thus, the energy of activation is lower.
Before breaking the R–X bond, the polar protic solvent also helps in the breaking by solvating the X and R. And after breaking of the R–X bond, the solvent stabilizes the anion and cation.
RX +H2O ⇋ R+ + X-
Effect of Nature of the Solvent in SN2 reaction:
Polar aprotic solvents have a crowded positive centre, so they do not solvate the anion appreciably. Hence, the rate of SN2 reactions increase when they are carried out in a polar aprotic solvent.
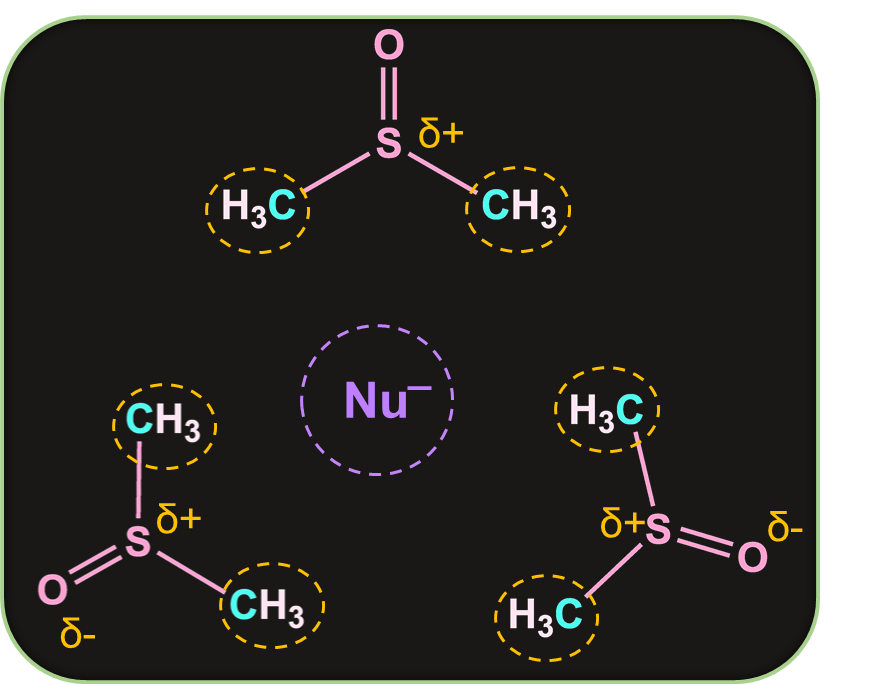
As we can see here, there is a repulsion between the methyls of DMSO and the nucleophile. As a result of which nucleophile remains unsolvated.
Note: DMSO and acetone are commonly used solvents in SN2 reactions.
Leaving group ability
Effect of Leaving Group Ability in SN1 reaction:
In the SN1 reaction, the leaving group begins to acquire a negative charge. Stabilization of this developing negative charge on the leaving group increases the rate of reaction, Hence good leaving groups favors the SN1 reaction.
The weaker the R-X bond, more easily it can be cleaved for the further nucleophile to attack.
|
Bond |
C-X Bond enthalpy (kJ mol-1) |
Bond length(pm) |
|
CH3-I |
234 |
214 |
|
CH3-Br |
293 |
193 |
|
CH3-Cl |
351 |
178 |
|
CH3-F |
452 |
139 |
Order of reactivity of R-X bond towards SN1 nucleophilic substitution is: R-I > R-Br > R-Cl >R-F
Effect of Leaving Group Ability in SN2 reaction:
A good leaving group stabilizes the transition state in SN2 reaction and thereby increases the rate of the reaction.
Order of reactivity of R-X bond towards SN2 nucleophilic substitution is: R-I > R-Br > R-Cl >R-F
Difference between SN1 and SN2
|
SN1 reaction |
SN2 reaction |
|
|
Order of reaction |
Unimolecular |
Bimolecular |
|
No. of steps involved |
Two or More |
One |
|
Nucleophile |
Weak nucleophile |
Strong nucleophile |
|
Solvent |
Polar protic solvent (Examples: Alcohols, water) |
Polar aprotic solvent (Examples: DMF, DMSO) |
|
Stability of alkyl halide(R-X) |
30 > 20 > 10 (requires the formation of a relatively stable carbocation) |
10 > 20 > 30 (requires unhindered substrate) |
|
Stereochemistry |
Partial Racemisation |
Inversion |
|
Leaving group |
-I > -Br > -Cl > -F for both SN1 and SN2 |
|
Practice Problems
1. Solvolysis of haloalkanes converts them into:
A). Acid
B). Ketone
C). Alcohol
D). Ester
Answer: C
Solution: If the solvent itself acts as a nucleophile, the reaction is known as solvolysis. In solvolysis water used as a solvent is water.
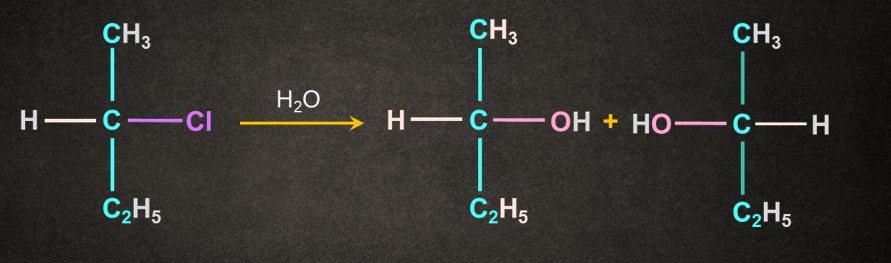
In this case water acts as both solvent and nucleophile. Haloalkanes when reacted with water give corresponding alcohols.
2. Which of the following solvent favors SN2 mechanism?
A). 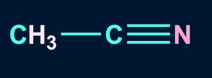
B). 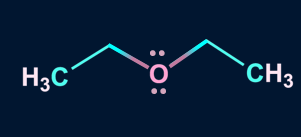
C). 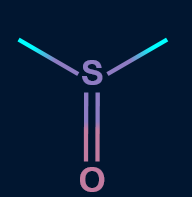
D). All of the above
Answer: D
Solution: Polar aprotic solvents have a crowded positive centre, so they do not solvate the anion appreciably. Hence, the rate of SN2 reactions increase when they are carried out in a polar aprotic solvent. Polar aprotic solvents do not have acidic hydrogen. Compounds in A, B, and C have no acidic hydrogen, thus are polar aprotic solvents.
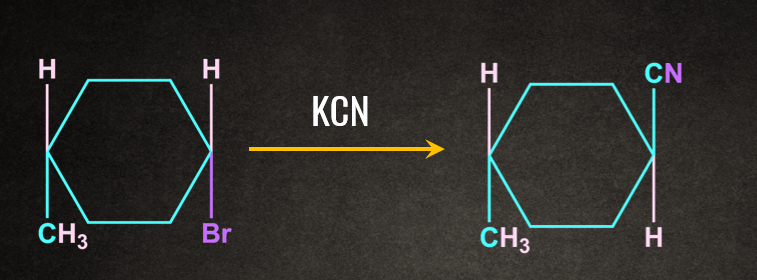
3. Find the major product of the following reaction?
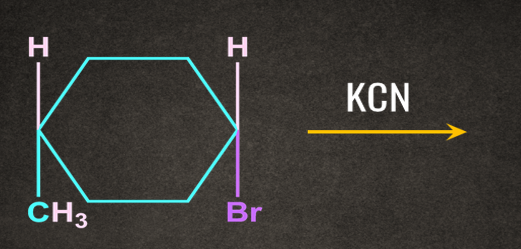
A).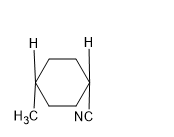
B). 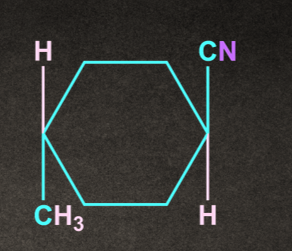
C). 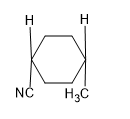
D). None of the above
Answer: B
Solution: SN2 reaction proceeds via formation of inversion of product.
Frequently Asked Questions - FAQs
1. What is a nucleophilic substitution reaction?
Answer: The replacement of an atom or group by any other atom or group in a molecule is known as a substitution reaction. If a substitution reaction is brought about by a nucleophile, then it is known as a nucleophilic substitution reaction.
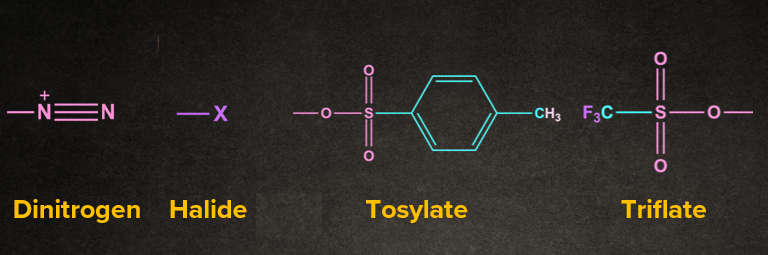
2. What is a leaving group?
Answer: The part of the reactant molecule which gets cleaved is called a leaving group. Generally poor bases are a good leaving group.
1. Nucleofuge: Leaving group that carries away an electron pair or negative charge. For nucleofuge, weaker bases are good leaving groups.
2. Electrofuge: Leaving group that comes away without an electron pair or having positive charge.
Generally, the leaving group carries away an electron pair.
Example:
3. What is racemisation?
Answer: When two enantiomers with chiral carbon have distinct rotations to plane polarized light are mixed in an equal amount, a racemic mixture is created.
4. What is polar protic solvent?
Answer: The solvent which has acidic hydrogen, i.e. exhibits H-bonding is a polar protic solvent.
5. Why halobenzene doesn’t give a SN1 reaction?
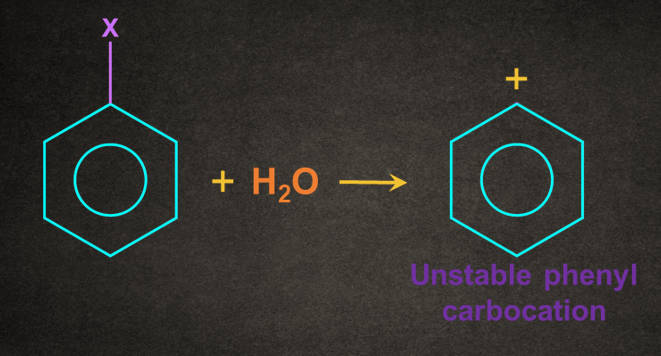
Answer: SN1 reaction of halobenzene gives phenyl carbocation which is highly unstable carbocation, generally not formed at room temperature. Therefore, halobenzene doesn’t give a SN1 reaction.