-
Call Now
1800-102-2727
ENTHALPY - Introduction, Enthalpy Change, Mathematical Representation, Enthalpy Change in Chemical Reactions, Standard Enthalpy Change, Practice Problems, FAQs
You might have heard about good, and bad conductors of heat, metal and wood respectively as examples. What makes them different? They have different abilities to take on the heat energy supplied to them and so also on passing out of it. On absorbing heat energy, the system’s energy increases. If you cool the system below its normal temperature, the opposite effect of cooling and lowering of energy occurs.
What this means is that when you give a certain amount of heat, there is a resulting rise in temperature that depends on the object.
The same thing happens at work. Don't you feel exhausted after work? It is because of a loss of energy.
Similarly, work on the system may increase the energy of the system, while work done by the system shall decrease its energy.
The first law of thermodynamics relates the energy changes (increase or decrease) of the system with the heat and work involving the system as follows.
dq = dU +dw: where dq is the heat changes, dU is the change in the internal energy of the substance and dw is the changes in the work done. A negative or positive sign is attached to the variables ‘dw’ depending on whether work is done on the system or by the system and similarly to dq whether heat is removed or given to the system. The net changes in the heat energy is called either internal energy or enthalpy. Let us go into knowing more about this fundamental property enthalpy here.
Table of contents
- Introduction to Enthalpy
- Enthalpy and Enthalpy Change
- Mathematical Representation of Enthalpy Change
- Enthalpy Change in Chemical Reactions
- Standard Enthalpy of Reaction
- Practice Problems
- Frequently Asked Questions
Introduction to Enthalpy
Heating the substance can be done under two conditions-namely isochoric or isobaric.
Changes in the heat and work done to result in changes in the temperature of the system. The changes can be brought about under two conditions either constant volume (isochoric process) or constant pressure (isobaric).
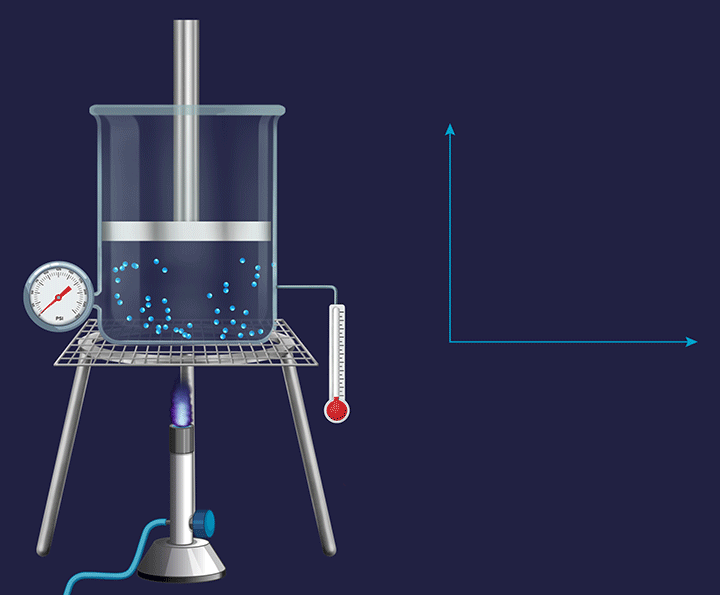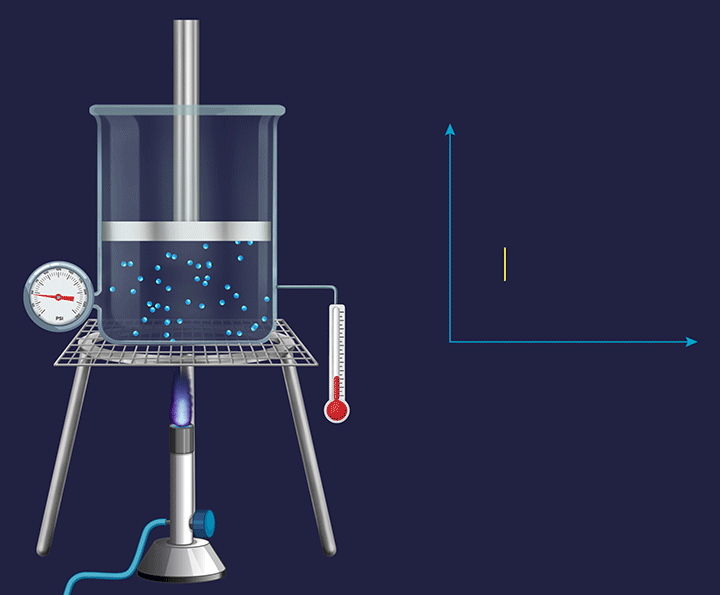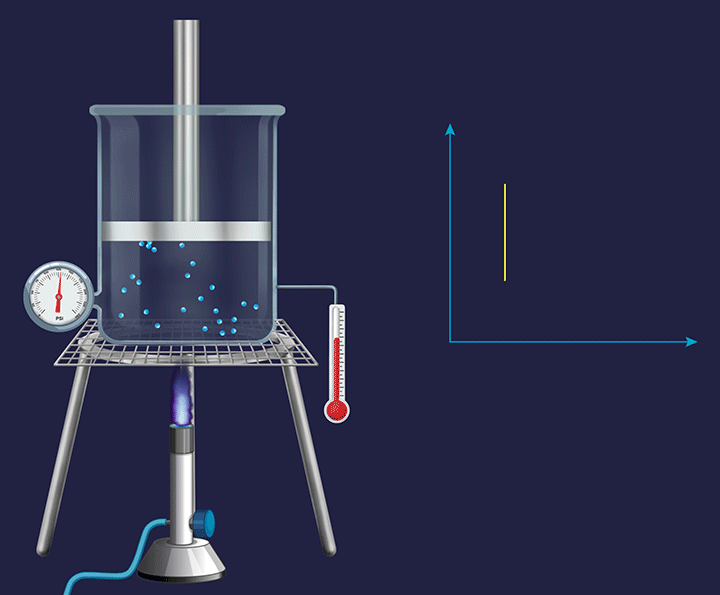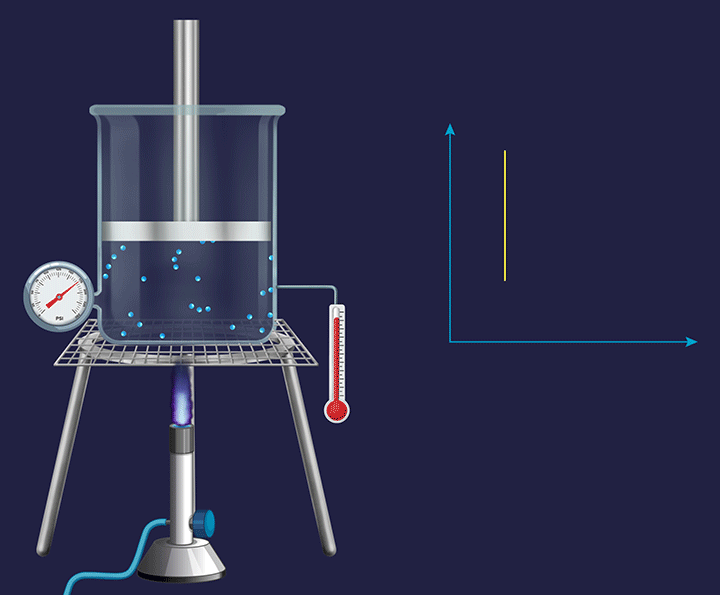
A system with a fixed piston attached to it has its volume fixed and any change occurring in such a system becomes an isochoric( constant volume during the experiment) process.
![]()
A system with a movable piston allows the volume to change to keep with the constant external pressure. Changes occurring in such a constant pressure system are called isobaric process.
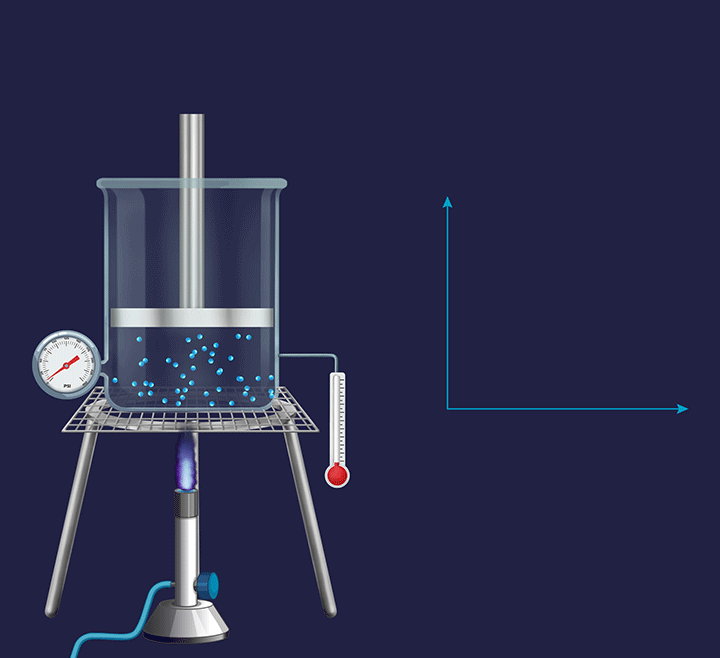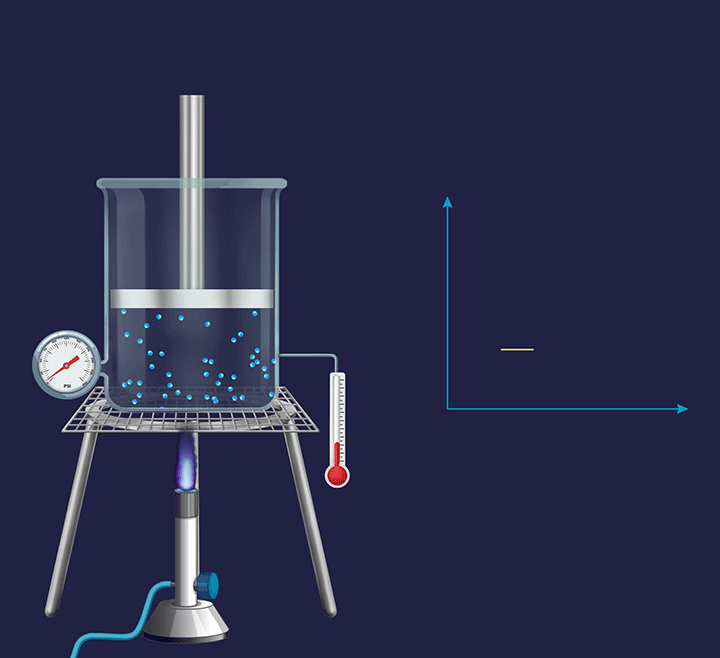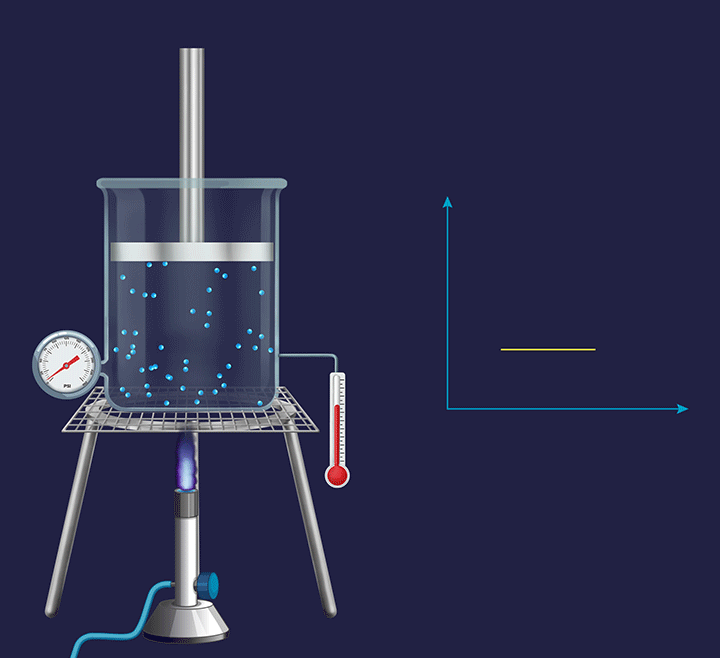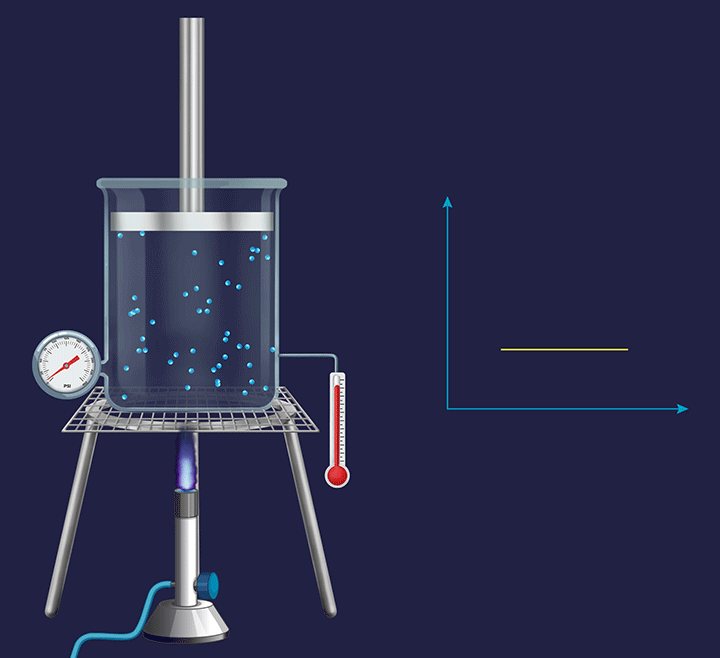
![]()
Thus, the internal pressure of the container during heat or work changes is constant, making the process isobaric.
So yes, in short, we have one system with an isochoric process and another with an isobaric one.
Let us take the first system - the isochoric one.
In chemistry, we talk about the volume change during the expansion or contraction of gases or during a change in the gaseous state of substances. So, we only consider the external work to be only a work of expansion or contraction measured by PdV.
Rewriting the equation for the first law for change in internal energy while heating.
dU= dQ-PdV
Since there is no change in volume in an isochoric system, therefore dv=0 and therefore -PdV=0
So, dU = dQ
Or, upon integration, U= QV where this QV stands for heat under constant volume conditions.
Therefore, the total heat taken up is fully retained as the internal energy of the system.
That is, if say 5J of heat is given to this system, then the internal energy of the system will be increased by 5J.
Now let’s consider the second system - the isobaric one.
Let’s give some heat to this system with the freely movable piston.
Since the piston is free to move here, the heat given is used up in increasing the internal energy as well as in doing some work, pressure volume work in this instance - by changing the volume of the system.
For instance, say the same 5J heat energy is given as before. Out of 5J, 3J of it might have been used up to increase the internal energy, while the remaining 2J would be used for the work of expansion done by the system.
So, out of these two systems here, given the same amount of Q to them,
The system undergoing the isochoric process will show higher temperature rise or a higher stored “heat content” comparatively because all the heat supplied goes into the internal energy component whereas the isobaric process expends part of this supplied heat as PV work.
Note the new term “Heat Content”.
To deal with this “heat content” of a system, we use another new term - Enthalpy H.
Enthalpy and Enthalpy Change
Enthalpy is the heat energy required to increase the internal energy and work to be done by the thermodynamic system at constant pressure.
In practice, the absolute value of the heat content or enthalpy of a substance or system is not needed. We are interested only in what happens to heat changes or enthalpy changes of the system during reactions or temperature changes.
Mathematical representation of Enthalpy Change
Mathematically, it is equal to the sum of internal energy, and the product of pressure and volume.
That is H=U+ PV
H=U+ PV
On differentiating, the equation can be rewritten like this
dH= dU+PdV+VdP
But by rearranging the first law equation, we know that dU+PdV=dQ
dU+PdV=dQ
So, dH= dQ+VdP
At constant pressure, there won’t be any change in P so dP will be zero. So the VdP term becomes zero and so dH will be equal to dQ, and H will be equal to Q.
At constant pressure, dP = 0
So, dH=dQ
Therefore, H=Q (at constant pressure)
In other words, enthalpy change is equal to the change in heat energy content of a system at constant pressure.
H= Qp
Where Q at constant pressure is denoted by this Qp
Enthalpy change in Chemical Reactions
Enthalpy change is the standard enthalpy of formation, which has been determined for a vast number of substances. In any general chemical reaction, the reactants undergo chemical changes and combine to give products. It can be represented by the following equation:
![]()
As enthalpy deals with volume changes, it is applicable to systems accompanying larger volume change during any transformation-like either formation or disappearance of gaseous molecules during reaction. Generally volume changes are minimum or nil in case of all reactants and products in either solid or liquid form.
For any chemical reaction involving gaseous molecules and carried out constant pressure (atmospheric pressure), the change in enthalpy is represented as ΔrH and is termed as the reaction enthalpy. The reaction enthalpy is calculated by subtracting the total enthalpies of all the reactants from that of the products.
Mathematically,
ΔtH = Sum of enthalpies of the product – Sum of the enthalpies of the reactants.
![]()
Here, the constants ai and bi denote the stoichiometric coefficients of the products and the reactants respectively for the balanced chemical reaction under consideration.
Endothermic and Exothermic Reactions and Sign Conventions
An isobaric chemical reaction releasing energy (mostly heat) along with the product is called exothermic reactions. The amount of energy releases is denoted by H equal to a negative numeral value. The opposite is true for an endothermic reaction which needs energy for the reaction.
The amount of energy changes is equal to the energy differences between the reactants and products and can be represented as follows.
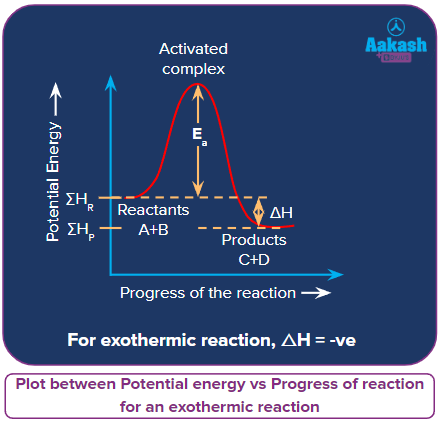
for an exothermic reaction.
In the exothermic reactions, the enthalpy during the conversion of reactants to products, decreases i.e.,
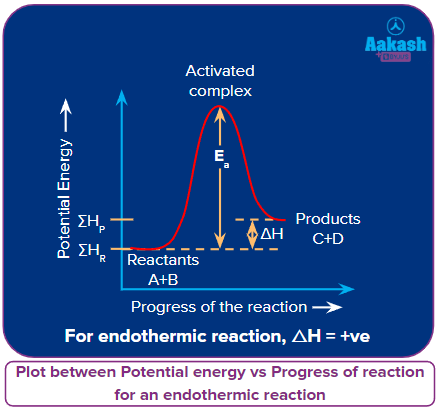
for an endothermic reaction.
In the endothermic reactions, the enthalpy during the conversion of reactants to products, increases i.e.,
Standard Enthalpy of Reaction
We already know the enthalpy of any reaction depends on the physical conditions of the surrounding such as the temperature, pressure, etc. In order to specify the standard enthalpy of any reaction, it is calculated when all the components participating in the reaction i.e., the reactants and the products are in their standard form. Therefore the standard enthalpy of reaction is the enthalpy change of a reaction involving all the reactants present in standard conditions.
As per convention, the standard state for any substance at a specified temperature is its pure form at a pressure of 1 bar. For example, liquid ethanol at 298 K and 1 bar Pressure is said to be in its standard state in its pure form. It is important to note that the data for the standard state for a substance is taken at 298 K. The standard enthalpy of a reaction is denoted as rHo. At constant pressure, the heat of the reaction is exactly equal to the enthalpy change of the reacting system.
Practice Problems:
Q1. The reactions which can not occur spontaneously are:
- Exothermic
- Endothermic
- Isothermic
- None of the above.
Answer: (B)
Solution: Endothermic reactions absorb heat from the surroundings, so this reaction will occur only when the heat will be provided. So they do not occur spontaneously.
Q2. Which of the following is an example of an endothermic reaction?
- Reaction of carbon with oxygen forming carbon dioxide
- Melting of ice
-
Burning of fuels
-
None of the above
Answer: (B)
Solution: The melting of ice is the process, where ice melts into the water at its melting point i.e., 0 ℃. This melting process happens when the ice absorbs the heat from the surrounding. When the heat is absorbed, the force that holds the molecules together weakens up and solid ice is converted into liquid water, the process of absorbing heat from the surroundings is known as the endothermic process. In all other options, heat energy is being released making them exothermic processes.
Q3. Which of the following reactions is an endothermic reaction?
A) C+O2→ CO2
B) N2+O2 → 2NO
C) 3H2+N2→ 2NH3
D) PCl3+Cl2 → PCl5
Answer: (B)
Solution: An endothermic reaction is the type of process where an increase in the enthalpy of the system occurs. Enthalpy change of those reactions is positive. The formation of NO is an example of an endothermic reaction as energy will be required to break the triple bonds in N2.
Q4. Calculate the standard heat of formation of carbon disulfide, given that the standard enthalpy of combustion of carbon (s), sulphur (s), and carbon disulfide (l) are respectively 390.0, 290, and 1100.0KJmol-1.
Solution:
Given …………(1)
…………..(2)
………….(3)
Use equation : (1) +2(2)-(3)
Adding the above three equations, we get-
Frequently Asked Questions – FAQs
Q1. Why is enthalpy useful?
Answer: Enthalpy is important because it informs us how much heat is in a system (energy). Heat is important since, from it, we can derive valuable work. An enthalpy shift shows us how much enthalpy was lost or obtained in terms of a chemical reaction, enthalpy meaning the system’s heat energy.
Q2. What is the difference between enthalpy and internal energy?
Answer: The cumulative energy that is stored in the device is internal energy. It is the amount held by the mechanism of potential and kinetic energy. Enthalpy is specified as the amount of the system’s internal energy plus the work done. Essentially both are energy content of the system but measured one at constant pressure and another at constant volume conditions.
Q3.What happens when enthalpy is positive?
Answer: A reaction having positive enthalpy needs energy for it to proceed. Energy has to be externally supplied or sometimes taken from the surrounding environment in nature, In which case, the temperature of the atmosphere falls.
Q4. What is the enthalpy of a chemical reaction?
Answer: The bonds between atoms can dissolve, reform or both during chemical reactions to either absorb or release energy. The heat absorbed or emitted under constant pressure from a device is referred to as enthalpy, and the reaction enthalpy is the change in enthalpy arising from a chemical reaction.
Q5. Is enthalpy always greater than internal energy?
Answer: The heat energy produced is often used in thermodynamics to improve the system’s energy or to do some useful work. The energy associated with an open system is called enthalpy, which is often greater than or equal to internal energy.


