-
Call Now
1800-102-2727
Effect of Halogens on Benzene Ring - Directive Influence of substituent, Practice Problems and FAQs
Assume your friend Raghav found a fantastic chemistry tutor in your city. Raghav is the only student who has visited this tutor. After a month, you also began working with the same chemistry tutor. Now, Tutor begins comparing your performance to Ragahv's performance. However, prior to your arrival, the tutor had no one to compare Raghav's abilities.

The same thing happens in the electrophilic substitution of halogen on benzene. Benzene can direct the first halogen substituent to any of its positions, but after monosubstitution, the position of the second substituent is determined by the first one. This is referred to as the Directive influence of a substituent on benzene.
Table of Contents:
- Introduction
- Directive Influence of substituent on benzene
- Effect of halogens on benzene rings
- Practice Problems
- Frequently Asked Questions
Introduction:
Can you answer these queries?
1. Are halogen-substituted benzenes ortho para or meta directing?
2. Do they deactivate the benzene ring or activate the monosubstituted benzene ring?
3. Which mono-substituted halobenzene/haloarene is the most reactive for an electrophilic substitution reaction?
When discussing the impact of halogen on the benzene ring, these three questions are the most perplexing.
In order to eliminate all of your doubts, all you need to do is seriously study and learn the concept below.
Directive Influence of substituent on benzene:
As previously stated, all six positions in the benzene ring are equivalent. As a result, any substitution that replaces any one of these six positions always results in a single monosubstituted product. When monosubstituted benzene is further substituted, the group already present on the benzene ring influences the incoming new groups.
The directional influence of groups refers to the capability of an existing group in the benzene ring to guide an incoming group to a particular position.
A substituent that is already on the ring has two effects:
(i) Orientation effect -. The three disubstituted products, ortho, meta, and para, do not form in equivalent amounts. The substituent on the benzene ring influences where the second substitution will be placed. Some groups direct substitution predominantly to the ortho and para locations, whereas others primarily to the meta position. It has been found that each group can be categorized as an ortho and para director or a meta director as a consequence.
(ii) Reactivity - Some substituents activate the ring, increasing its reactivity in comparison to benzene. The ring is deactivated by several substituents, which lowers the ring's reactivity in comparison to benzene. For instance, a -OH group increases the ring's reactivity compared to benzene, whereas a -NO2 substituent decreases the ring's reactivity.
The groups can be categorised into three groups:
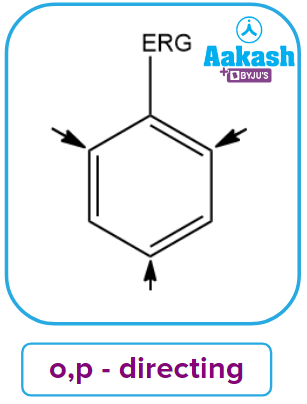
- ortho- and para-directing Activating groups: These groups activate the benzene ring by donating electrons. These groups are known as electron-releasing groups (ERG). These groups assign ortho and para locations to the incoming groups. The typical examples include
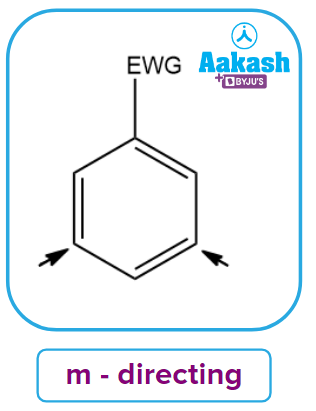
- Meta-directing Deactivating groups: These groups deactivate the benzene ring by withdrawing electrons. These groups are known as electron withdrawing groups (EWG). These groups assign meta locations to the incoming groups. The typical examples include
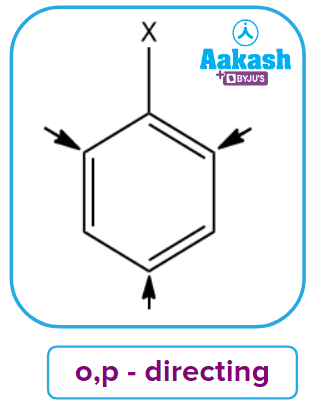
- ortho- and para-directing Deactivating groups: These groups cause the benzene ring to be deactivated and to withdraw electrons by -I effect. These groups assign ortho and para locations to the incoming groups. The typical examples include:
Effect of halogens on benzene rings:
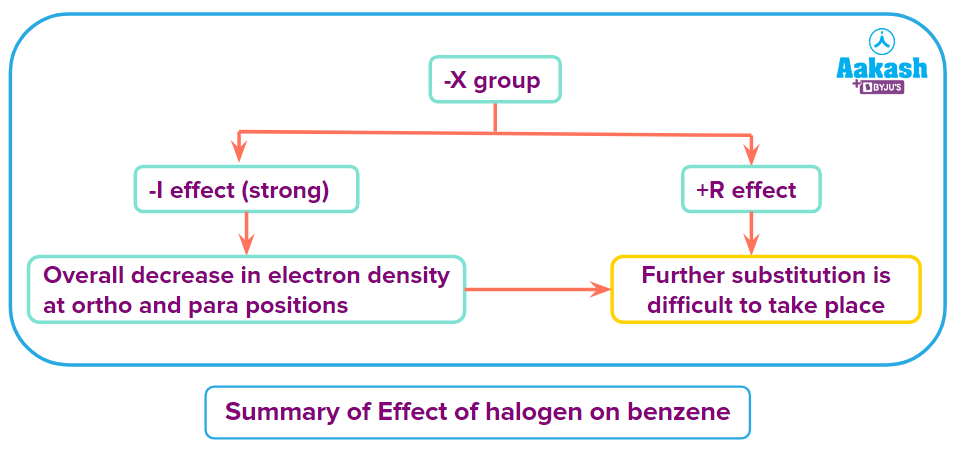
Halogens exhibit the +R and -I effects. Halogens are somewhat deactivating groups because of the aryl halides' significantly stronger -I action compared to +R effects. However, the ortho-para positions see a greater rise in electron density from the +R effect than the meta position.
Electrophilic substitution is difficult because the electron density at the ortho and para positions has often reduced in comparison to benzene.
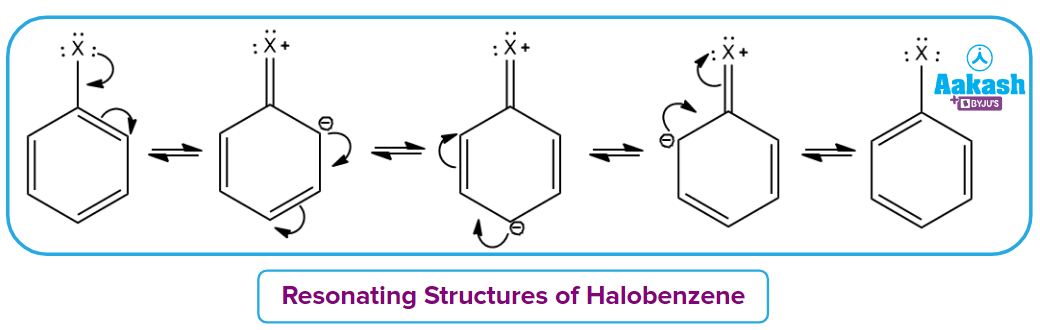
Directive influence of halogen on benzene:
Due to the stronger -I effect, the overall electron density on the benzene ring decreases but the orientation of the second group in benzene is decided by the resonance effect. So, due to the +R effect of halogens, they are ortho and para directing.
Practice Problems:
Q1. What will be major products A and B in the following reaction?
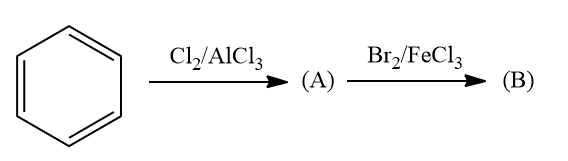
A. Chlorobenzene, 1-Chloro-2-Bromobenzene
B. Chlorobenzene, 1-Chloro-3-Bromobenzene
C. Reaction will not occur
D. Chlorobenzene, 1-Bromo-4-Chlorobenzene
Answer: D)
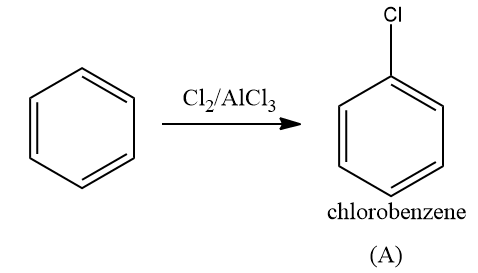
Solution: Arenes react with halogens in the presence of a Lewis acid like anhydrous FeCl3, FeBr3, or AlCl3 to yield haloarenes. Hence as halogen is chlorine, product A is chlorobenzene.
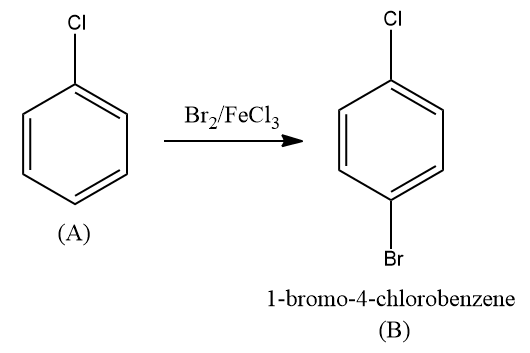
The electron density at the ortho-para positions is increased more than the electron density at the meta position by the halogen’s +R effect. Product B needs to be an ortho or para product as a result. Due to less steric restriction, the para product is more stable than the ortho product. Therefore, 1-Bromo-4-Chlorobenzene is the main product.
Hence, the correct answer is option (D).
Q2. Which of the following monosubstituted benzene will be least reactive towards electrophilic substitution reactions?
(A) 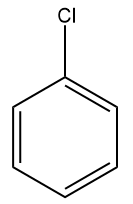 (B)
(B) 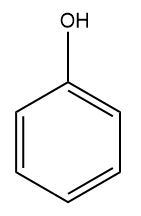
(C) 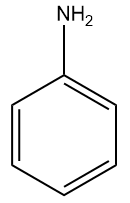 (D)
(D) 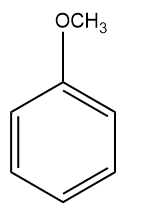
Answer: (A)
Solution: For electrophilic substitution, the group should be electron-releasing in nature. There are two types of ortho para-directing groups, one is ortho- and para-directing activating groups which cause the benzene ring to be activated and to release electrons. For example: and other are ortho- and para-directing deactivating groups which cause the benzene ring to be deactivated and to withdraw electrons. For example -F,-Cl,-Br,-I etc.
It is clearly understood that the Chlorine group being deactivating group (dominating –I effect) in chlorobenzene will decrease the reactivity towards electrophilic substitution reactions compared to other groups which are activating groups. Hence, the correct answer should be an option (A).
Q3. Which of the following will be more reactive to halobenzene's electrophilic aromatic substitution?
(A)  (B)
(B) 
(C) 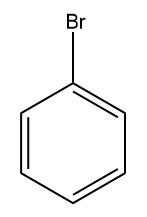 (D)
(D)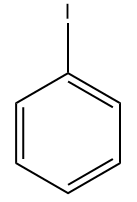
Answer: (A)
Solution: If we consider the resonating structure of halobenzene, it can be clearly understood that for electrophilic substitution reaction,
 In the case of Fluorobenzene, the overlapping of 2p orbital carbon and 2p orbital of fluorine is pretty good as compared to another halobenzene which has 3p orbital in Chlorobenzene, 4p orbital in Bromobenzene and 5p orbital in Iodobenzene respectively.
In the case of Fluorobenzene, the overlapping of 2p orbital carbon and 2p orbital of fluorine is pretty good as compared to another halobenzene which has 3p orbital in Chlorobenzene, 4p orbital in Bromobenzene and 5p orbital in Iodobenzene respectively.

Due to the greater resonance contribution from this effective electron-donating resonance interaction with fluorine, It is more reactive than another halobenzene. Hence, the correct answer should be option (A).
Q4. Which of the following monosubstituted benzene will be most reactive towards electrophilic substitution reactions?
(A) 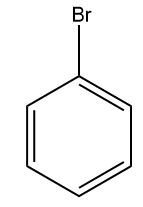 (B)
(B) 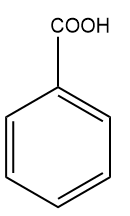
(C) 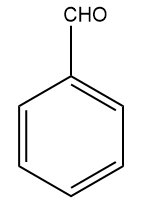 (D)
(D) 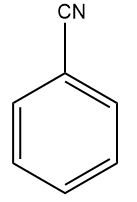
Answer: (A)
Solution: For electrophilic substitution, the group should be electron-releasing in nature. There are two types of deactivating directing groups which withdraw the electrons, one is meta-directing deactivating groups which cause the benzene ring to be deactivated and to withdraw electrons by the -R effect. For example and others are ortho- and para-directing deactivating groups which cause the benzene ring to be deactivated and to withdraw electrons by -I effect. For example,-F,-Cl,-Br,-I etc.
We all know that the group with the -R effect will withdraw more electrons as compared to the -I effect. So, the ortho- and para-directing deactivating groups Br will deactivate least as compared to meta-directing deactivating groups; -COOH,-CHO,-CN . Hence, the correct answer should be an option (A).
Frequently Asked Questions-FAQs:
Q1. What are some applications for halo-substituted benzene?
Answer: Chlorobenzene is mostly used as an intermediary in the manufacturing of products including rubber, dyestuffs, and herbicides. Additionally, the production of adhesives, paints, paint removers, polishes, dyes, and pharmaceuticals uses chlorobenzene as a high-boiling solvent.
Q2. What distinguishes haloalkanes from haloarenes?
Answer: Haloalkanes are substances formed when a halogen atom replaces the hydrogen atoms in aliphatic hydrocarbons. Similar to this, compounds are known as haloarenes when halogen atoms replace hydrogen atoms attached to benzene ring.
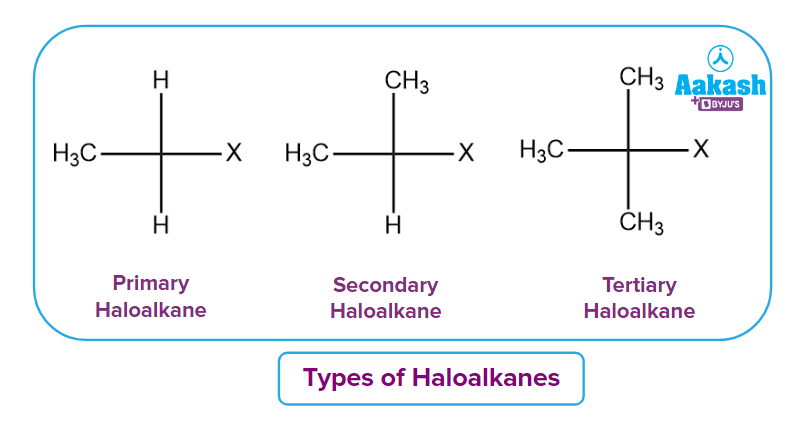
Q3. How do haloalkanes differ from one another?
Answer: On the basis of the carbon atom to which the carbon-bearing halogen (X) atom is linked, they are further divided into three basic types: primary, secondary, and tertiary.
Q4. Why is Haloarene difficult for nucleophilic substitution reactions?
Answer: In comparison to haloarene, haloalkane has a longer C—Cl link. Haloarenes are less reactive than haloalkanes toward the nucleophilic substitution process because shorter bonds are more difficult to break than longer bonds.


