-
Call Now
1800-102-2727
Chemical Kinetics - Rate of a Reaction, Factors Affecting the Rate of a Reaction, Molecularity, Order, Differential Rate Law & Integrated Rate Law
You must have seen the speedometers in the vehicle. Can you tell me what the significance of the speedometer is?

A speedometer in the vehicle helps to determine the speed of the vehicle and it is calculated by calculating the distance covered by the vehicle in a particular time interval.
But can you tell me how can we determine the speed of the chemical reaction?
There is a branch in chemistry that deals with the rate or speed with which the reaction is taking place and is named Chemical Kinetics.
Peter Waage and Cato Guldberg initiated the development of chemical kinetics in 1864 when they proposed the law of mass action. This law states that the speed at which chemical reaction is taking place is proportional to the activity of reacting substances each raised to the power of their respective coefficients. Let's learn in this article about some important concepts related to the rate of reaction and the importance of chemical kinetics.
Table of Contents
- Introduction to Chemical Kinetics
- Rate of a Reaction
- Instantaneous Rate of a Reaction
- Average Rate of Reaction
- Factors Affecting Rate of Reaction
- Molecularity of Reaction
- Order of Reaction
- Differential Rate Law of Reaction
- Integrated Rate Equation
- Importance of Chemical Kinetics
- Practice Problems
- Frequently Asked Questions-FAQs
Introduction to Chemical Kinetics
Chemical kinetics is known as reaction kinetics, which studies the rates of the reactions and how they are affected by different factors like- temperature, the concentration of reactants, the presence of catalysts etc. It also aids in gathering and analysing information about the reaction's mechanism and defining the characteristic feature of a chemical reaction. It contrasts with thermodynamics, which deals with the path of a process, and the feasibility of the process on the basis of energy and also helps in determining the efficiency, but does not tell us anything about its rate.
For example,
Graphite is more stable than diamond which can be explained by thermodynamics and due to which the graphite can be converted into diamond with time but how long it will take to convert graphite is determined by chemical kinetics. Diamond is kinetically more stable than graphite and therefore the conversion process will be very slow.
Rate of a Reaction
The change in concentration of the reactants or products per unit time is defined as the rate of a reaction. It is the rate at which reactants are converted into products.
Let’s consider a reaction, ,
The rate of reaction for the above chemical reaction can be expressed in two ways:
Rate of decrement in the concentration of the reactant which is also known as the rate of disappearance of reactant. Mathematically,
Rate of disappearance of reactant (A) =
The rate of increment in the concentration of product formed in the reaction is also known as the rate of appearance of the product. Mathematically,
Rate of appearance of the product (B) =
Rate of disappearance of the reactant, rate of appearance of the product and rate of reaction can be related with the help of stoichiometric coefficient of the reactant/product. Mathematically, the Rate of reaction can be represented as:
Rate of reaction =
Rate of reaction =
Note: The negative sign signifies the decrease in the concentration of the reactant as the rate of reaction is always a positive value.
Instantaneous Rate of a Reaction
The change in concentration in an infinitesimally small time interval is defined as the instantaneous rate of a reaction.
Let’s assume that the term 'Δt' (time interval) is very small and almost tends to zero. We have an infinitesimally small 't' that can be considered as at a particular instant of time. Mathematically instantaneous rate of a reaction can be represented as
Instantaneous rate of a reaction
‘C’ represent the concentration of the reactant.
Graphical representation of the instantaneous rate of a reaction
In the concentration vs time graph, the instantaneous rate of a reaction at any time can be determined by calculating the slope of the tangent at a particular time on the curve.
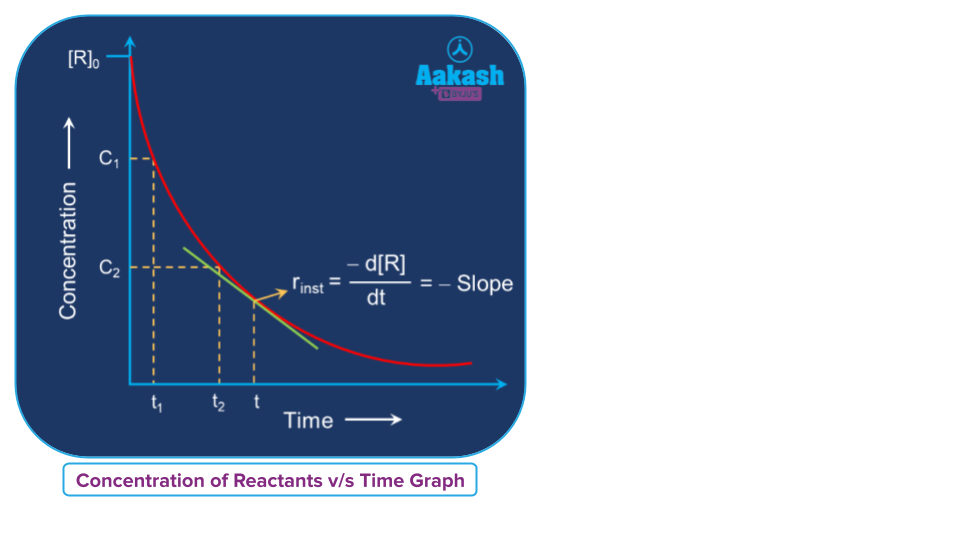
Instantaneous rate of a reaction (rint) = Slope of the tangent at a specific point on the curve =
Average Rate of a Reaction
The average rate of a reaction is defined as the change in concentration over a significant time interval. Mathematically,
Let’s consider a reaction,
Average rate of reaction
Average rate of disappearance of reactant ‘A’ can be represented as:
Average rate of disappearance of 'A'
Average rate of appearance of 'B' can be represented as:
Average rate of appearance of 'B' =
During the reaction the concentration of the product increases, therefore,
Note: As the stoichiometric coefficient of the reactant and product is equal to one, therefore, it can be said that;
Average rate of reaction (ravg)= Average rate of disappearance of 'A'= Average rate of appearance of 'B'
Graphical representation of the average rate of a reaction
In a concentration vs time plot the average rate of a reaction between any two intervals can be calculated by determining the slope of the line using straight-line equation by connecting those two points in a given time interval.
Let’s consider the chemical reaction,
Let R1 and P1 be the concentrations of reactant or product at time t1
Let R2 and P2 be the concentrations of reactant or product at time t2
Plot of [R] vs time
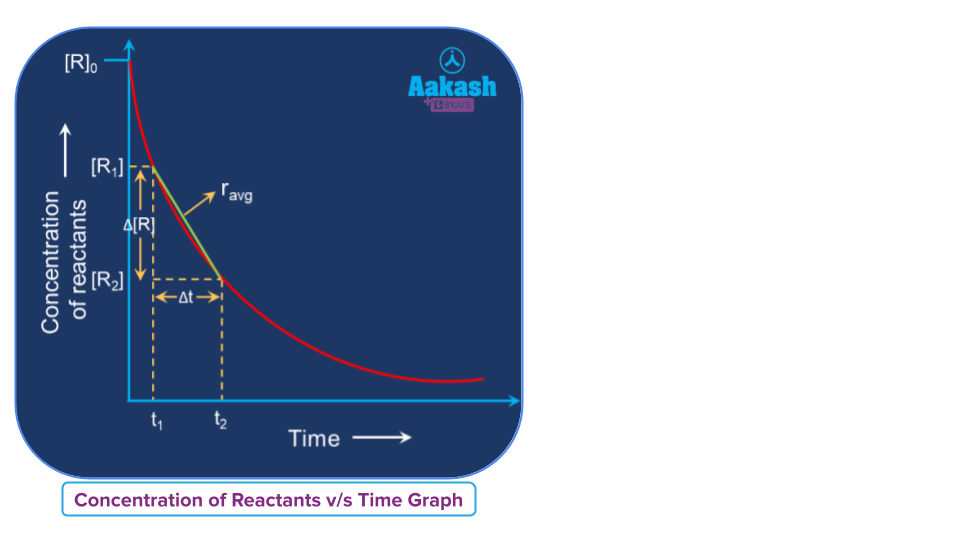
The average rate of a reaction (ravg) = Slope of the straight line joining two points 1 and 2
Note: Negative sign here signifies the decrease in the concentration of the reaction.
Factor Affecting the Rate of a Reaction
There are several important factors that affect the rate of reaction which includes:
Concentration of reactant: Reactants collide with each other to form products, according to collision theory, which will be discussed later. The number of colliding particles increases as the concentration of reactants increases, increasing the rate of reaction.
Nature of reactant: The rate of the reaction is also affected by the types of substances that are reacting. The reaction that occurs during the formation of a covalent bond between molecules, which results in the formation of larger molecules, is usually slower. Furthermore, the nature and strength of bonds in reactant molecules affect the rate of their transformation into products significantly.
Physical state of reactants: The physical state of a reactant, whether solid, liquid, or gas, can have a significant impact on the rate of change. The reaction takes place primarily at their point of contact. For example, in the case of a liquid and a gas, at the liquid's surface.
Surface area of reactant: If we take two solids, the particles at the surface will participate in the reaction. Similarly, if we crush a solid into smaller pieces, more particles will be present at the surface. This means that the number of collisions between these and reactant particles will almost certainly increase. As a result, the reaction will happen faster.
Temperature: The number of collisions between reactant molecules per second increases as the temperature rises (frequency of collision). Increases, causing the reaction rate to increase. However, whether the reaction is endothermic or exothermic, increasing the temperature increases the rate of forward or backward reactions.
Effect of solvent: The nature of the solvent is also affected by the rate of reaction of the solute particles.
Catalyst: Catalysts change the reaction mechanism, which changes the rate of the reaction. In general, a catalyst increases the rate of reaction by decreasing the activation energy required for the reaction to proceed. In the case of inhibitors which is a negative catalyst, the rate of reaction decreases as the activation energy increases by adding negative inhibitors.
Molecularity of Reaction
It is defined as the number of reacting molecules colliding at the same time to produce a chemical reaction. In other words, it is the number of reactant molecules involved in a simple reaction.
Consider the following reaction:
The molecularity in this reaction is two as one atom of A collides with one atom of B to form AB molecule.
The molecularity of a reaction is not valid in the case of complex reactions (i.e. those reactions which take place in a multiple-step). It is only valid in the case of elementary reactions (i.e. reaction taking place in a single step) and is equal to the stoichiometric coefficient of the reactant involved in the reaction.
The molecularity of reaction above four is generally not observed because the probability of four atoms colliding together to form a product is very less.
Order of Reaction
The relationship between the rate of a chemical reaction and the concentration of the species involved is referred to as the order of the reaction.
The order of the reaction is defined as the rate's power dependence on all reactant concentrations.
Consider this reaction:
For this reaction, the rate of reaction (R), can be represented as:
So, the order of reaction with respect to A is 1, the order of reaction with respect to B is 1 and overall order of the reaction is 2.
The following are some characteristics of a chemical reaction's reaction order:
- It represents the factor by which the rate of reaction is dependent on the concentration of reactant present in a given chemical reaction.
- Reaction’s order is not affected by the stoichiometric coefficients of each reactant in the balanced chemical reaction and is experimentally determined but in the case of elementary reaction order of the reaction is equal to the stoichiometric coefficient of the reactants.
- A chemical reaction's order is defined by the concentration of reactants, not on the concentration of products.
- The order of reaction can have an integer, fraction and negative value. It can be even zero.
- Sum of the order of all the reactants present in the rate law expression gives the total order of the reaction.
On the basis of the order of the reaction, reactions are classified as:
- Zero order reactions
- First order reactions
- Second-order reactions
Differential Rate Law Equation
According to the rate law, the rate of the chemical reaction is proportional to the activity of the reactants raised to the power some coefficient.
The activity of the reactant is proportional to the concentration of the reactant and the coefficient is determined experimentally. In the case of elementary reactions, the coefficient is equal to the stoichiometric coefficient of the reaction.
Let’s consider a chemical reaction,
According to the rate law and considering the reaction to be the elementary reaction,
Rate of reaction ∝ [A]a [B]b
Where a and b are the stoichiometric coefficients of reactants A and B .
Rate of a reaction [B]b
Here,
- [A] & [B] are the concentrations of the reactants A and B respectively.
- a and b are the orders with respect to the reactants A & B respectively which is equal to stoichiometric coefficient a and b respectively as the reaction is an elementary reaction.
- ‘k’ represents the proportionality constant also known as the rate constant for the reaction.
The above equation is known as a differential rate equation.
Note:
- Rate constant for the reaction is only temperature-dependent, which changes with a change in temperature.
- The rate law expression for the same reaction may differ under different conditions such as temperature and pressure.
Integrated Rate Equation
A differential rate equation represents how the rate of reaction is dependent on the concentration of reactants. Tangent’s slope at any point in time on the graph (Concentration vs Time) represents the instantaneous rate of reaction and determining the rate of reaction using a concentration-time graph is extremely difficult. As a result, the differential rate equation is integrated to obtain a relationship between concentration at various points and the rate constant. This is known as the integrated rate equation. Based on the order of the reaction the rate of reaction is related to the concentration of the reacting species and then integrated to find the time required for the reaction to complete.
Importance of Chemical Kinetics
Chemical kinetics finds a vast range of applications in industries as well as in the laboratory. Some important application of chemical kinetics includes:
- It is concerned with the investigation of reaction rates and mechanisms which help in identifying the time required for the completion of the reaction.
- It helps to identify the impact of catalysts and temperature on the rate/speed of chemical reactions.
- During the cracking of heavy hydrocarbons into lighter gases and gasoline, through kinetic models it can be determined that on what temperature and pressure the yield would be maximum.
- It can be used in the modification or design of chemical reactors to improve product yield, separate products more efficiently, and remove environmentally damaging by-products.
- It aids in the understanding and description of chemical processes such as food decomposition, microorganism growth, and stratospheric ozone decomposition.
Recommended video: https://www.youtube.com/watch?v=xWvnxyWRuB0
Practice Problems
Q1. Which of the statements is correct for the order of reaction?
- Order of reaction can have any value including zero, fraction and negative.
- Order of reaction is experimentally determined.
- Order of reaction in the case of elementary reaction is equal to the sum of the stoichiometric coefficient of reactants.
- All of the above
Answer: (D)
Solution: Order of reaction can be defined as the power dependence of rate on all reactant concentrations. The order of a reaction can be a positive, negative, fraction or zero and is determined experimentally. In case the elementary reaction order of the reaction is equal to the sum of the stoichiometric coefficient of all the reactants present in a given reaction.
So, option (D) is the correct answer.
Q2. Select the correct statement with respect to the molecularity of the reaction.
- Molecularity of zero-order reaction is 0.
- Molecularity of reaction is defined as the number of atoms colliding with each other to form the product.
- Molecularity of reaction can have any value including zero, fraction and negative.
- Molecularity of the reaction is determined experimentally.
Answer:(B)
Solution: Molecularity is defined as the number of reacting molecules colliding at the same time to produce a chemical reaction. In other words, it is the number of reactant molecules involved in an elementary reaction. The molecularity of the reaction can only have a positive integral value and is a theoretical concept. The molecularity of the reaction can only have a positive integral value as it represents the number of atoms that collide in a proper orientation to form the product. Therefore, option (B) is correct.
Q3. If for a reaction: , which is a 2nd order with respect to the reactant ‘A’. Select the correct option from the following.
- Rate of reaction = ( [A] represents the initial concentration of reactant)
- If the concentration of reactant is doubled the rate of reaction becomes 4 times
- It is an example of an elementary reaction.
- Order and molecularity of the reaction are the same for this reaction
Answer: (B)
Solution: According to the given question,
AB is a 2nd order reaction and essentially a complex reaction as the order of the reaction is not equal to the stoichiometric coefficient of the reaction. As the reaction is a complex reaction of 2nd order therefore the molecularity of the reaction cannot be determined.
The rate of the reaction can be mathematically represented as,
Rate of reaction =
Here,
[A] represent the concentration of reactant at time ‘t’
‘k’ represent the rate constant of the reaction
Since the given reaction is 2nd order when the concentration of the reactant is doubled the rate of reaction becomes four times. Therefore option (B) is correct.
Q4. Select the correct option for the factor which is responsible for the change in the rate constant of the reaction?
- Pressure
- Concentration of the reactants
- Surface area
- Temperature
Answer: (D)
Solution: Rate constant of the reaction is only dependent on the temperature and is independent of the other factors like- pressure, the concentration of reactants, surface area etc. Whereas the rate of reaction depends on the factors like temperature, nature of reactant, the concentration of reactants, pressure, surface area etc. Therefore, option (D) is correct.
Frequently Asked Questions-FAQs
Q1. Why do we say that a zero-order reaction is essentially a complex reaction?
Answer: Zero-order reaction is the type of reaction in which the rate of reaction is independent of the concentration of the reactant. It is essentially a complex reaction because if we consider the reaction to be elementary it is not possible to form a product without any reactant.
Q2. What is the difference between the rate constant and equilibrium constant?
Answer: The rate constant of a reaction is the proportionality constant calculated for the reaction moving in a particular direction and is equal to the ratio of the rate of the reaction to the concentration of the reactants each raised to the power of some coefficient (determined experimentally). The rate constant of the reaction is determined experimentally.
The equilibrium constant of the reaction is calculated for the reversible reaction and is the ratio of the concentration of the products each raised to the power stoichiometric coefficients to the concentration of reactant each raised to the power stoichiometric coefficients. It can also be calculated by taking the ratio of the rate constant of forward reaction to the rate constant of backward reaction.
Q3. What is the difference between molecularity and the order of the reaction?
Answer:
|
Molecularity of reaction |
Order of reaction |
|
It is defined as the number of reacting molecules colliding at the same time to produce a chemical reaction. In other words, it is the number of reactant molecules involved in a simple reaction. |
The order of reaction can be defined as the power of the concentration on which the rate of the reaction depends |
|
The molecularity of the reaction is determined theoretically. |
The order of the reaction is experimentally determined. |
|
The molecularity of the reaction can only have a positive integral value. |
The order of the reaction can be positive, negative or zero. It can also have a fractional value |
|
Molecularity is defined only for the elementary reaction and has no significance for complex reactions. |
The order can be calculated for both elementary as well as for complex reactions. In the case of elementary reaction, the order is equal to the sum of the stoichiometric coefficient of all the reactants. |
Q4. How to determine the order of the reaction in case of a complex reaction?
Answer: The order of reaction can be defined as the power dependence of rate on all reactant concentrations. It is experimentally determined for the reaction but in the case of elementary reaction experimentally determined entity is equal to the stoichiometric coefficient of the reactant.


