-
Call Now
1800-102-2727
Chemical Bonding - Definition, Theories, Formation, Types, Examples, Practice problems & Frequently asked questions
Look at the picture given below and try to observe what this girl is doing?
Putting some papers together and trying to make something? Yes, you guessed it right. But now think, if she will be able to keep all the papers intact in one place in a way she wants just by keeping them together? No. For this, she needs something which can hold everything together to get the desired structure and to keep them bound. So, for a paper she needs glue.
In chemistry also, we need such a kind of glue which can hold different particles like atoms, molecules, ions etc together so that we can achieve a stable structure of chemical compounds. This bonding in chemistry is known as chemical bonding.
Let’s dig deeper into it to understand the concept of chemical bonding.
TABLE OF CONTENT
- Chemical bonding
- Kossel-Lewis’s electronic theory
- Why are chemical bonds formed?
- Types of chemical bonding
- Practice problems
- Frequently asked questions - FAQs
Chemical bonding
Chemical bonding is the formation of a chemical bond between two or more atoms, molecules, or ions that results in the formation of a chemical compound. These chemical bonds are what hold the atoms together and make them stable in the resulting compound.
Kössel and Lewis's Electronic theory
In 1916, Albrecht Kössel and Gilbert Lewis were the first to successfully explain the formation of chemical bonds. The inertness of noble gases was used to explain chemical bonding.
Before understanding the Lewis and Kossel approach, let’s first understand the octet rule.
- Octet rule
The octet rule states that the atoms are most stable when their valence shells are filled with eight electrons. To achieve inert gas configuration, the atoms lose, gain or share electrons.
• It has been observed that atoms of noble gases have little or no tendency to combine with each other or with atoms of other elements.
• It means that these atoms must have a stable electronic configuration.
• These elements (noble gases) have eight electrons () in their outermost shell, except the helium that has two electrons (
) in the outermost shell.
Given below are the examples of the elements with 8 electrons in their outermost shell and are considered as stable.
Now, as we are clear about what the octet rule is, let’s understand Lewis's approach of chemical bonding
According to lewis approach of chemical bonding, atoms achieve the stable-octet configuration by sharing electrons.
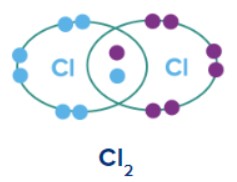
The Lewis approach can be understood by considering the formation of the chlorine molecule, . The
atom with electronic configuration,
is one electron short of the argon configuration.The formation of the
molecule can be understood in terms of the sharing of a pair of electrons between the two chlorine atoms, each chlorine atoms contributing one electron both to the shared pair. Thus each chlorine atom gets argon configuration and forms single bond between chlorine atoms.In this process, both chlorine atoms attain the outer shell octet of the nearest noble gas (i.e., argon). Such structures are referred to as Lewis dot structures.
Let’s now see Kössel’s approach of chemical bonding
Kössel explained the formation of ionic bond. The bond formed by the transfer of electrons from highly electropositive atom to highly electronegative atom is known as ionic or electrovalent bond.
• The bond formed, as a result of the electrostatic force of attraction between the positive and negative ions, was termed as the electrovalent bond or ionic bond.
• The electrovalence is thus equal to the number of unit charge(s) on the ion. For example, In case of , chlorine has an electrovalency of 1.
Why are chemical bonds formed?
Let’s understand this with the help of an example.
Looking at electronic configuration of sodium and chlorine
=
If will lose one electron it will attain stable noble gas configuration and will become
=
If will gain one electron it will attain stable noble gas configuration and will become
We can clearly see that is losing one electron to become positively charged and
on the other side is gaining one electron to become negatively charged. Both the ions will then due to electrostatic forces of attraction between the charges will form ionic bond which will hold
and
together.
So, it’s clear by looking at this example that the reason why chemical bonds are formed is that whenever a chemical bond is formed it leads to the stability of an atom or a molecule by attaining a stable electronic configuration.
The strength of the chemical bonds between its constituents influences the stability of the resulting compound; the stronger the bonding between the constituents, the more stable the resulting compound.
The inverse is also true: if the chemical bonding between the constituents is weak, the resulting compound will be unstable and will easily undergo another reaction to produce a more stable chemical compound (containing stronger bonds). Atoms try to lose energy in order to find stability.
Chemical bonds are formed not just by losing or gaining electrons as shown above but there are many types of chemical bonds which help atoms to attain stability. Many of them have been given below.
Types of chemical bonding
Chemical bonding is of two types, intramolecular and intermolecular, which can further be divided into different categories as given below:
1. Intramolecular bond: Intramolecular bonds are the chemical bonds that are present between atoms of a molecule or compound. There are three types of intramolecular bonds:
2. Intermolecular bond: Intermolecular bonds are attractive forces between two neighboring particles (atoms, molecules or ions). They are significantly weaker than intramolecular bonds. There are three types of intermolecular bonds:
Practice problems
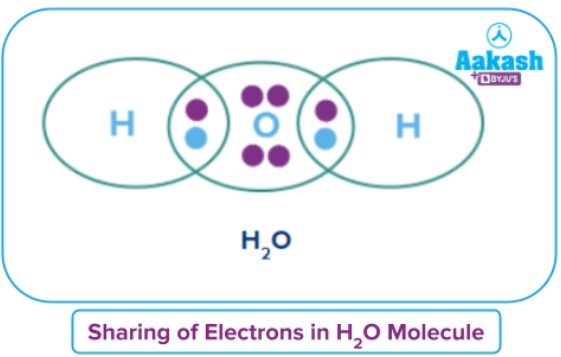
Q1. Draw lewis dot structure of .
Answer: We know,
Electronic configuration of = 2, 6
Electronic configuration of = 1
So, need two more electrons to complete its octet whereas
need 1 more electron to complete its duplet and become stable.
Two atoms will come and bonded with
and share one electron each with
thereby completing its octet and oxygen also share one electron with one
atom thereby completing its duplet as shown in the given structure.
Q2. Why do noble gas elements like Helium (He), Neon (Ne), Argon (Ar) Krypton (Kr), and Xenon (Xe) do not react whereas all other elements can?
Answer: =
=
=
=
=
If we look at the electronic configuration of these noble gases, we can observe that they have completely filled valence shells i.e., they are already stable. Due to this reason they do not react with any other other element.
Q3. Describe how B and Al break the octet rule?
Answer: Boron and aluminium, both from Group 13, have different bonding properties. Because these atoms each have three valence electrons, we can predict that they will want to bond covalently in order to gain 5 electrons through sharing and thus fulfill the octet. However, compounds with five bonds formed by boron or aluminium atoms have never been observed, so we must conclude that simple predictions based on the octet rule are unreliable for Group 13 and octet rule is not applicable for all the elements.
Q4. In a compound, can elements of that compound be written randomly?
Answer: In general, the least electronegative atom occupies the central position in the molecule/ion.
For example; In , carbon is the central atom whereas oxygen occupy the terminal positions
Q5. or
, which is stronger and stable?
Answer: We know thatthestronger the bonding between the constituents, the more stable the resulting compound. Also, the fact that ionic bonds are stronger than covalent bonds we can say that is stronger and stable as compared to
due to the formation of ionic bond.
Q6. Why Hydrogen does not follow octet rule?
Answer: Electronic configuration of H =
1st shell of an atom can accommodate maximum 2 electrons and hydrogen only need one electron to become stable so formation of octet in this case is not possible. Instead of octet, hydrogen forms duplet.
Frequently asked questions-FAQs
Question 1. Why do atoms react and how?
Answer: Atoms combine to form molecules to lower the energy by forming chemical bonds. The atoms which do not have octet configuration try to achieve it by forming chemical bonds by losing, gaining or sharing electrons.
Question 2. Kossel and Lewis predicted what kind of chemical bond?
Answer: Both Kossel and Lewis predicted the formation of an ionic bond and covalent bond.
Question 3. Do all elements follow the octet rule?
Answer: No, all elements do not follow the octet rule.
For example; Hydrogen only requires one additional electron to achieve the stable configuration, whereas lithium must lose one electron by ionically combining with other elements. As a result, when hydrogen and lithium bond to other elements, they both have two electrons in their valence shell i.e., they are completing duplet—the same electronic configuration as helium which shows that they don’t need to complete their octet to attain stable electron configuration.
| Ionic bonding | Chemical formulae of common compounds |
| Covalent bonding | Limitations of octet rule, |
| Metallic bonding | Coordinate bonding |


