-
Call Now
1800-102-2727
Applications of Henry's Law: Henry’s Law, Applications, Practice Problems & Frequently Asked Questions

You might have seen in pictures and cinema, about scuba divers carrying small cylinders on their backs. Any idea about, the content of the cylinder?
Is that an oxygen gas tank to fortify the oxygen supply for breathing? If so, it raises many more questions like,
What pressure does the gas cylinder have? Is it only pure oxygen or a mixture? Is it safe to use such cylinders? Is there anything to be followed in using such external supplies?
But they are very important questions to be answered because it will be a matter of life and death for the scuba divers. Definitely, you will be interested to know about the answers.
To answer all these questions let us understand a principle underlying these activities, namely Henry’s Law.
Table of Contents:
- Henry's Law
- Applications of Henry’s Law
- Practice Problems
- Frequently Asked Questions
Henry’s Law:
You know that molecules evaporate from any liquid at all temperature and pressure conditions to form a vapour. The vapour exerts pressure on the walls of the container and on the liquid. The amount of vapour and the pressure exerted by the vapour, called vapour pressure depends on so many things like temperature, pressure, nature of the solute/ solvent etc.
Henry’s Law, quantifies the vapour pressure, over a liquid, or a gas in terms of the amount present in the solvent.
It states that “the partial pressure of the gas in the vapour phase (p) is directly proportional to the mole fraction of the gas (x) in the solution” and is expressed as
Where KH= Henry’s law constant

Henry’s Law constant varies with conditions, temperature, pressure, interactions between the gas and the solvent.
In simple terms, Henry's law states that the amount of the gas soluble or present in a liquid is proportional to the partial pressure of that gas existing above the liquid. Higher the vapour pressure, the higher the concentration of the gas inside the liquid.
The diagram below depicts the link between the solubility of a gas in a liquid and its partial pressure in the atmosphere above the liquid (as mandated by Henry's law).
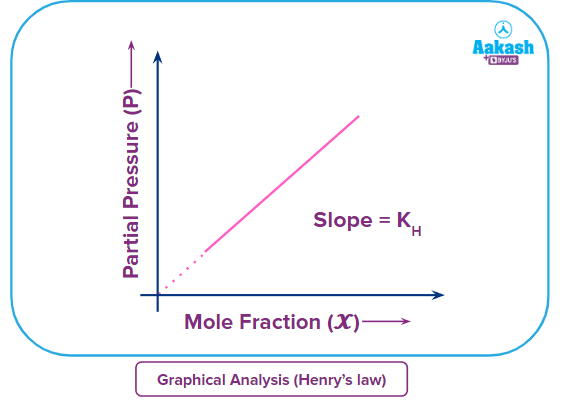
It can be seen from the graph that the mole fraction of the dissolved gases increases linearly with the external pressure over the liquid.
Applications of Henry’s Law:
1. Carbonated Soft Drinks:
Carbonated drinks have carbon dioxide gas under pressure in a container containing water. The gas that is dissolved in water in the container directly depends on the pressure inside the container.
On opening the container, the gas escapes out lowering the carbon dioxide pressure above the water. This allows some of the dissolved oxygen to become gaseous carbon dioxide making up some of the lost pressure due to the escape of the gas. Now the amount of gas in water will be less than that of what was dissolved under high pressure initially. If the bottle is left open, this process of dissolved gas coming out and escaping into the atmosphere will happen continuously till the exhaust of any carbon dioxide in the water.
2. In Respiration:
Henry's law is most commonly used to understand the distribution of oxygen and carbon dioxide in the bloodstream and alveoli. Due to atmospheric pressure oxygen dissolves in blood in alveoli and supplies to the cells. The pressure of oxygen gets reduced and is compensated by the dissolving of exhaled carbon dioxide from the cell. Carbon dioxide is 22 times more soluble than oxygen and hence taken away to be released in alveoli. The process continues in the uptake of oxygen and removal of carbon dioxide.
3. Climbers:
As you climb the mountains, the pressure of oxygen in the air diminishes. The partial pressure of oxygen in the air at very high elevations is substantially lower than at ground level. As a result, persons who live at high altitudes or climb have low oxygen concentrations in their blood and tissues. This causes weakness and a loss of mental clarity. Anoxia is a condition caused by these symptoms.
4. Scuba diving:
The pressure in the bloodstream rises as the scuba diver descends further and deeper into the sea. When a diver inhales high-pressure air from the cylinder, the bloodstream absorbs also the gaseous nitrogen present in the air.
According to Henry's Law, as pressure rises, so does nitrogen solubility in the diver's blood. This stops nitrogen from escaping from the compressed air in the bloodstream until it may escape through low-pressure exhalation, as he or she ascends to the surface. This requires a slow and steady ascent to the surface for the gradual release of blood nitrogen. You can imagine what will happen in case of a fast ascent to the surface.
Unfortunately, divers knowingly or unknowingly rise too quickly, causing nitrogen bubbles in the blood to form quickly. It obstructs capillaries and induces a condition known as curvature. This is extremely painful and perhaps fatal. To minimise twisting and the poisonous effects of excessive levels of nitrogen in the blood, scuba divers now utilise air diluted with helium in their cylinders (11.7% helium, 56.2% nitrogen and 32.1% oxygen).
Practice Problems:
Q.1. At 25o C , the CO2 Henry's Law constant is Determine the mole fraction of CO2 at a 1.5 atm pressure.
Solution: We know that,
Q.2. Find Henry's law constant for CO2 dissolved in water at 298 K. The mole fraction of CO2 in 1 L soda water when packed under 2.5 atm pressure at 298 K is
Solution: Given
Therefore,
We know that
Substituting the values of P and x,
Q3. HCN is a toxic gas used in qualitative analysis. If the solubility of HCN in water at STP is 0.175 M, what is the value of KH?
Solution:
Number of moles of
Number of moles of
Mole fraction of
Pressure at STP
According to Henry’s law,
Q4. KH for carbon dioxide in water at 298 K is. What is the amount of carbon dioxide in 1 L of water. The pressure of carbon dioxide is 2.5 atm at 298 K.
Solution: We know that
Frequently Asked Questions:
Q1. Why does Henry's Law only apply to dilute solutions?
Answer: Henry's law is a limitation that only holds true in the case of diluted solutions. The range of concentration in which it applies narrows the more the system deviates from the ideal behaviour. The concentration of a solute in diluted solutions is approximately inversely proportional to its mole fraction. This is a logical conclusion because diffusion will occur at a higher rate with steeper concentration gradients. Here, the partial pressure of a gas in its gaseous state and its equivalent mole fraction in its solution state serve to indicate the solute's concentration.
Q2. Why does Henry's Law hold true at high temperatures and not at high pressures?
Answer: Henry's law does not apply to gases that operate at extremely high pressures. When the pressure rises, the pressure of atmospheric gases rises as well, therefore the solubility of nitrogen gas rises and becomes toxic when it enters the blood supply.
Only when a gas behaves like an ideal gas Henry’s law is applicable. Ideal gases are those in which there is no attraction between the gas molecules. When the pressure is high and the molecules are close to each other, the attraction between the gas molecules occurs. To overcome this attraction, the temperature is raised, causing molecules to travel faster.
Q3. What variables affect Henry's Law constant (KH)?
Answer:
1. Temperature and pressure: These two are the key factors of gas solubility in a liquid. The solubility of the gas will increase significantly as the temperature and partial pressure of the gas in the liquid rise. As a result, Henry's constant value is constantly changing as gas temperature and partial pressure fluctuate.
2. Nature of the gas: The nature of gas dissolved in the liquid has a major effect on the value of Henry's constant. Different gases have various Henry constants.
3. Solvent type: Henry's constants are also influenced by the kind of solvent used to dissolve the gas.
Q4. Why do oxygen and helium follow Henry's Law while hydrochloric acid does not?
Answer: Henry's law does not apply to HCl gas because it dissociates into the ions H+ and Cl- when it is in solution. Strong acids like hydrochloric acid (HCl) interact with their solvents and easily split up into their component ions. While oxygen and helium follow Henry's law because they do not dissociate when in contact with water.


