-
Call Now
1800-102-2727
Alkene: Alkene, Hydrogenation, Dehydrohalogenation, Dehalogenation, Dehydration, Physical properties, Uses, Practice Problems & FAQs
A boon for humans yet a boon for the environment, is what alkenes can be called when it is produced artificially. Civilisation and development has seen a manifold increase in the utility and production of alkenes and we, the global citizens at this point of time, have become extremely dependent on it.
But hey! Before considering alkenes to be only culprits, look at how it helps in making us naturally see the world so clearly!! Amazed? Yes, the chemistry of vision is dependent on a substituted alkene called Retinal, which is an oxidised version of Vitamin A (Retinol). They all contain multiple alkene groups. Rhodopsin, the major light-gathering pigment in the retinas of vertebrates, is created when the oxidised form of retinal, i.e., retinol. Rhodopsin is also essential for cell proliferation and the maintenance of healthy skin tissue. Hence naturally present alkenes are supporters of life!
Artificially prepared alkenes play an important role in our daily lives. It is an important part of our lives. We usually use alkenes most of the time. There are various applications and uses of alkenes. We mostly use plastics, such as bags, polythene, grocery bags, etc. All these contain alkenes in them. The major sources of these hydrocarbons are petroleum and coal. These are also used in cooking as fuel.
In this article, you will learn in detail about the preparation, properties, and uses of alkenes.
Table of Content
- Alkene
- Classification of Alkenes
- Hydrogenation of Alkenes
- Dehydrohalogenation of Alkenes
- Dehalogenation of vicinal dihalides
- Dehydration of Alcohols
- Physical Properties of Alkenes
- Stability of Alkenes
- Laboratory test of Alkenes
- Uses of Alkenes
- Practice Problems
- Frequently asked questions
Alkene
- Alkenes are unsaturated hydrocarbons containing at least one double bond.
- The general formula for alkenes is CnH2n.
- Alkenes are also known as olefins (oil-forming).
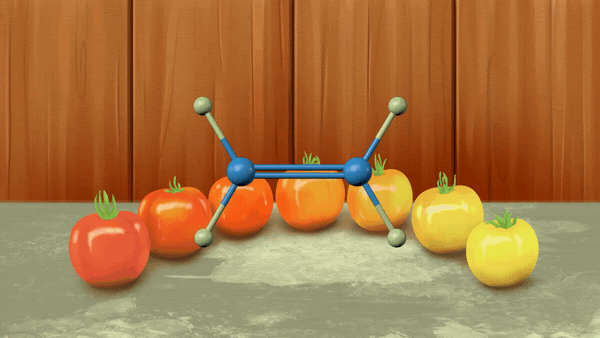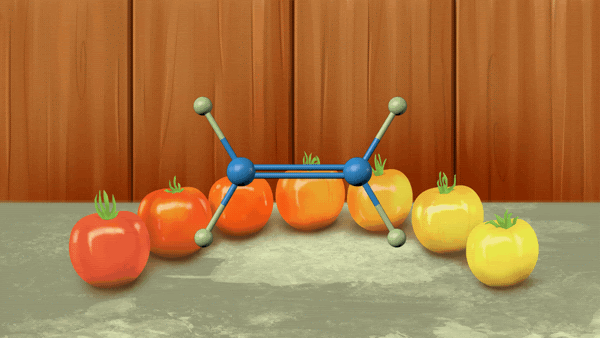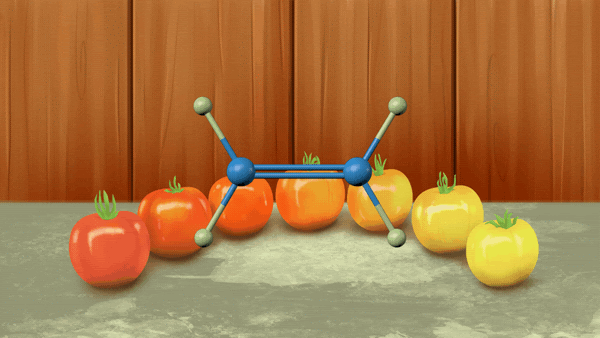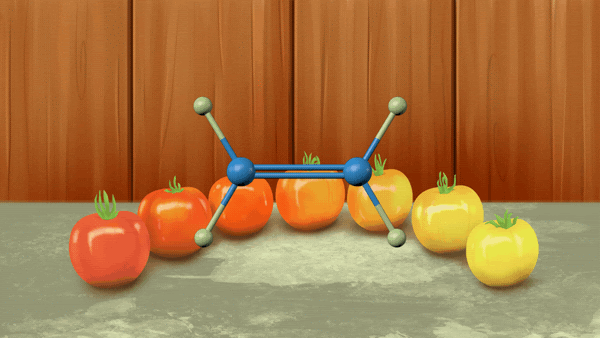
Classification of alkenes
Alkyl groups connected to the sp2 hybridised carbon atoms of alkenes have an impact on the stability of the double bond. The amount of alkyl groups attached to the sp2 hybridised carbon atoms can also change how reactive alkenes are chemically. Alkenes can then be categorised based on how many alkyl groups are linked to the C=C structural unit. This characteristic is known as the degree of substitution.
A single alkyl group is connected to the double bond's sp2 hybridised carbon atom in monosubstituted alkenes. An alkene containing a double bond at the end of the carbon atom chain is referred to as a terminal alkene. Alkyl groups are joined to the carbon atoms of the double bond in disubstituted, trisubstituted, and tetrasubstituted alkenes, respectively.
|
Types |
Formula |
|
Monosubstituted |
RCH = CH2 |
|
Disubstituted |
RCH = CHR |
|
Trisubstituted |
RCH = CR2 |
|
Tetrasubstituted |
CR2 = CR2 |
Relative Reactivity of Hydrocarbons
Alkenes and alkynes have weak π bonds containing loosely held electrons, which makes alkenes/
alkynes reactive towards electrophiles. Electrophiles are electron-loving species. So, alkenes and
alkynes show electrophilic addition reactions.
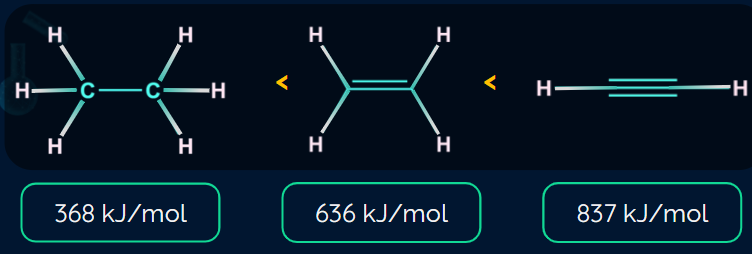
Bond Strength Of Hydrocarbons
A carbon-carbon triple bond (837 kJ mol-1) is stronger than a carbon-carbon double bond, which is stronger (636 kJ mol–1) than a carbon-carbon single bond (368 kJ mol-1).
Preparation of Alkenes
Generally, alkenes can be prepared by the following four methods:
- Hydrogenation of Alkynes
- Elimination reactions of Alkyl halides
- Elimination reaction of Vicinal dihalides
- Dehydration of Alcohols using conc. H2SO4
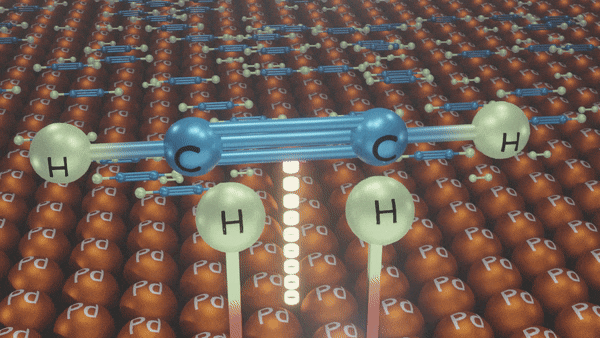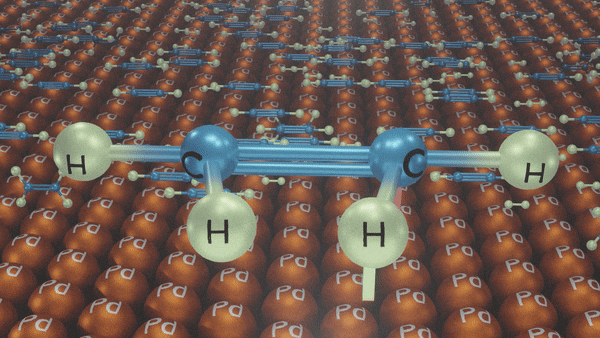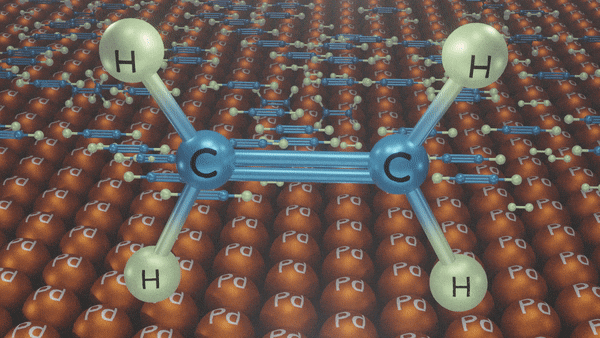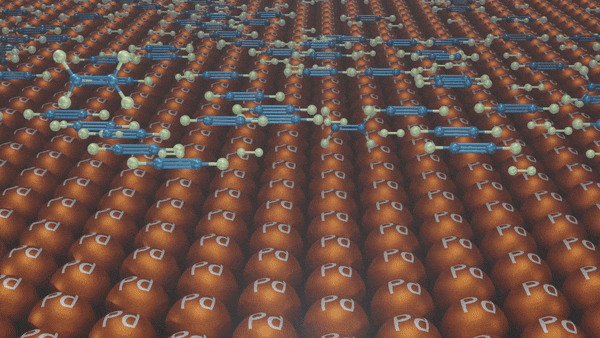
Hydrogenation of Alkynes
- Lindlar’s Catalyst
Reagents: H2/Pd, CaCO3, quinoline
Poisoned palladium catalyst: It is composed of powdered calcium carbonate coated with palladium and poisoned with quinoline to reduce its catalytic activity so that a complete reduction of alkynes does not take place. Lindlar’s catalyst is used to carry out partial reduction of alkynes to alkenes. Poisoning deactivates the catalytic activity to an extent and the reduction of the alkyne is restricted to the formation of an alkene.
Example:
In the presence of Lindlar’s reagent, only cis alkenes are formed.

Reduction of Alkynes using Lindlar’s Catalyst
- Birch Reduction
The conversion of alkyne to alkene using Na/liquid NH3 in the presence of EtOH is known as the Birch reduction. This is a trans-addition.
Alkynes give trans alkenes in the Birch reduction.
Example:
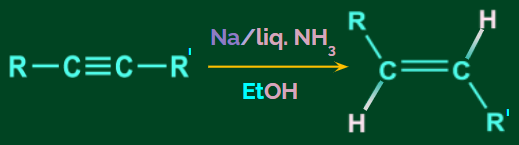
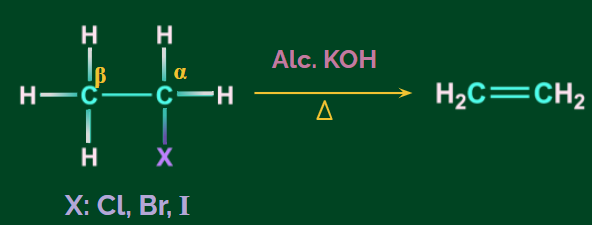
Dehydrohalogenation of Alkenes
- Alkenes can be prepared from alkyl halides by dehydrohalogenation, which means the elimination of HX.
- Reagent used is alcoholic KOH.
- β-Hydrogen is removed and is, therefore, known as the β-elimination reaction.
- The hydrogen opposite to the halogen atom attached to the β-carbon (carbon atom next to the carbon to which halogen is attached) is removed. So, it is known as anti-elimination.
Example:
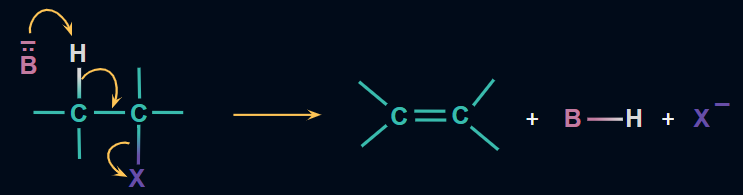
Mechanism:
The alkoxide ion sourced from the alcoholic KOH acts as a strong base. It attacks the β-H atom, which is slightly acidic in nature, and separates it from the alkyl halide molecule. The electrons shared by the broken hydrogen‐carbon bond are attracted towards the ⍺-carbon atom, which is slightly electron-deficient due to being attached to the halogen atom. As these electrons approach the ⍺-carbon atom, the halogen atom breaks, leading to the formation of the double bond.
Saytzeff or Zaitsev rule
In most elimination reactions, where there are two or more possible products, the predominant product will be the one with the highly substituted double bond.
Example:
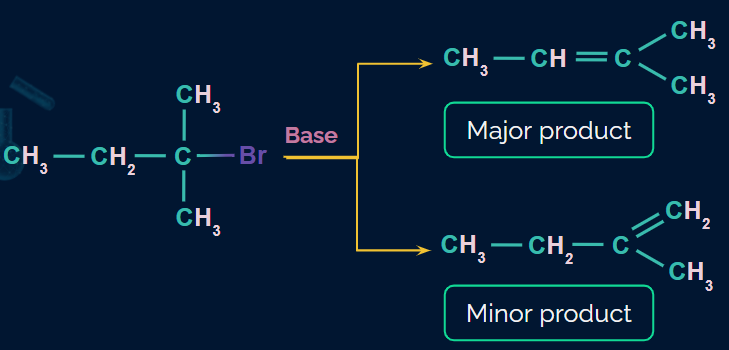
Dehydrohalogenation of RX
- Dehydrohalogenation, or the removal of one halogen acid molecule, occurs in this process. The kind of the connected halogen group and the alkyl group affect the pace of reaction.
- Reactivity: R−I > R−Br > R−Cl > R−F
- Greater the size of halide ion, weaker the H-X bond and greater its reactivity. Hence RI is most reactive. This is because iodide is better leaving group due to its large size.
Rate of elimination
- The rate of elimination reaction directly depends upon the stability of alkene.
- Rate: Tertiary alkyl halide > Secondary alkyl halide > Primary alkyl halide
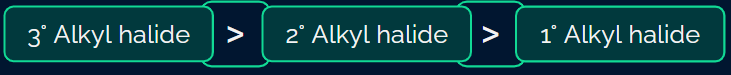
This is because tertiary halides have more number of β-hydrogens as compared to secondary and primary halides.
Dehalogenation of vicinal dihalides
- Alkenes are prepared from vicinal dihalides by dehalogenation, i.e., elimination of X2.
- The reagent used is NaI in acetone or Zn in the presence of acetic acid or ethanol or Zn/Δ.
- This reaction proceeds through the E2mechanism.
Reaction:
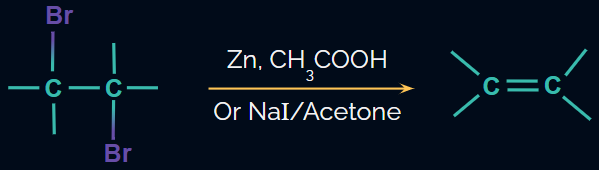
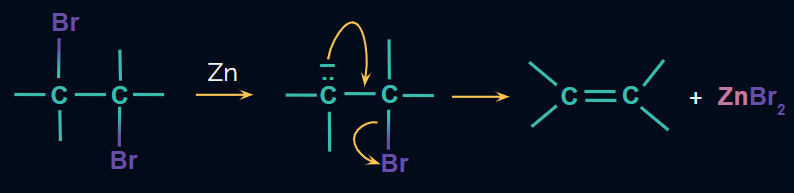
Mechanism:
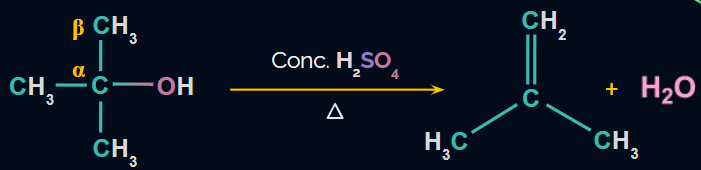
Dehydration of Alcohols
- Alkenes can be prepared from alcohol by acidic dehydration, i.e., the elimination of H2O in the presence of acid.
- The reagent used is conc. H2SO4 and heat.
- β-H is removed and therefore, it is known as the β-elimination reaction.
- The reaction proceeds through the E1 mechanism
- The reaction involves formation of carbocation, so rearrangement may take place.
Reaction:
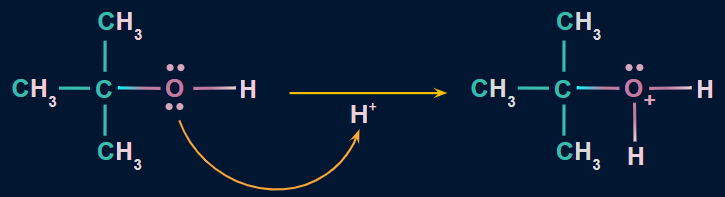
Mechanism
Step 1: Formation of a protonated alcohol
- Due to the presence of two lone pairs of electrons on oxygen, alcohols act as weak bases.
- Therefore, they react with strong mineral acids (H2SO4) to form a protonated alcohol.
- Protonation of alcoholic oxygen facilitates the elimination of water molecules.
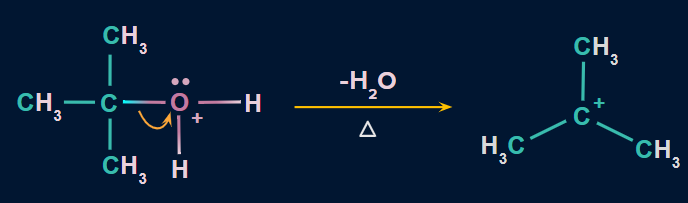
Step 2: Formation of carbocation
- In this step, the C−O bond breaks with the elimination of a water molecule to form carbocation.
- This is the slowest step. Hence, it is considered as the rate-determining step.
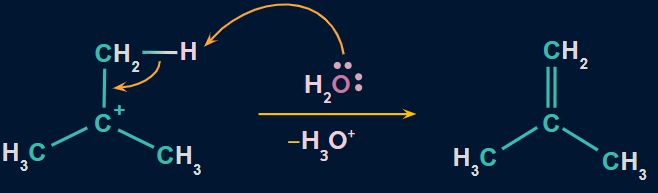
Step 3: Formation of alkene
- Here, water attacks the proton of the carbon atom adjacent to the carbocation, breaking the existing C − H bond to form C = C.
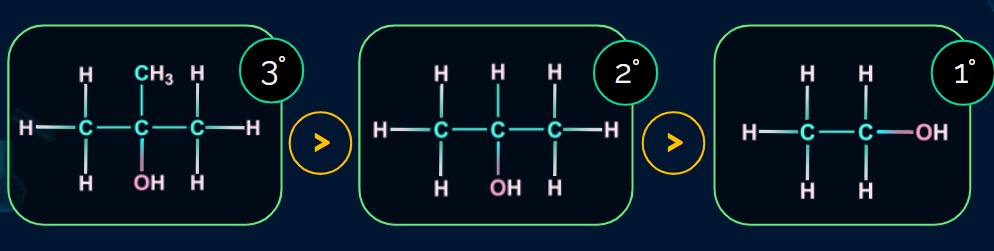
Order of reactivity of alcohols towards dehydration
Order of reactivity of alcohols towards dehydration:
Physical Properties of Alkenes
- Physical State of alkenes
|
No of Carbon atoms |
State |
|
2 - 4 |
Gases |
|
5 - 18 |
Liquids |
|
19 |
Solids |
- Color of alkenes
All alkenes are colorless.
- Odor of alkenes
All alkenes are odorless except ethene which has a faint sweet smell.
- Solubility of alkenes
Being non polar, alkenes are generally insoluble in water (polar) but soluble in organic solvents ( cyclohexane, benzene, etc.)
- Boiling Point of alkenes
- With the increase in the molecular weight, the Boiling point of alkene increases.
- Straight-chain alkenes have a higher boiling point than branched-chain alkenes due to increases in the van der waal forces by an increase in the surface area in straight-chain alkenes.
- Melting Point of alkenes
- With the increase in the molecular weight, the Melting point of alkene increases.
- For isomeric alkenes, the melting point of alkene depends on the symmetry of alkene. Alkene, which is more symmetrical in nature has a higher melting point.
- Example: Trans isomeric alkene is having higher melting point than Cis isomeric alkene.
- Density
The density of alkenes is less than water. These are lighter than water.
Stability of Alkenes
The stability of alkenes depends on two factors:
- Delocalisation of electrons
The greater the delocalisation of electrons in an alkene, the more stable the alkene is.
- Number of alpha hydrogens
The alkene with the maximum number of alpha hydrogens is the most stable.
Example :
- As 2,3-dimethylbut-2-ene contains a maximum number of alpha hydrogens(12 alpha hydrogen) is more stable than 2-methylbut-2-ene, it contains 9 alpha hydrogen, more stable than isobutylene as it contains only 6 alpha hydrogen.
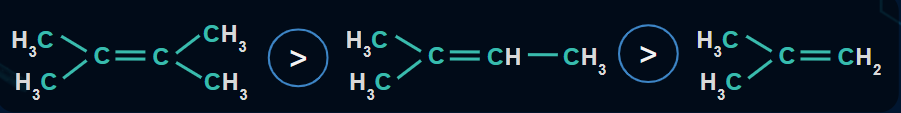
- Stability order of the compounds is given on the basis of delocalisation of electrons.
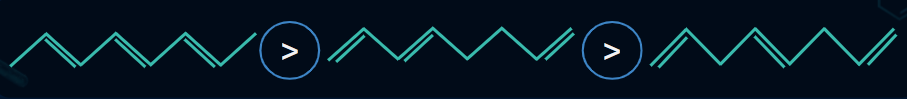
Bond Length of Alkenes
The Bond length of alkenes changes due to delocalisation of pi electrons and hyperconjugation.
Example:
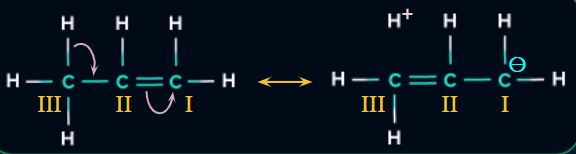
- Bond Length of C(II) - C(III) is less than normal C-C bond length.
- Bond Length of C(II) - C(I) is greater than normal C=C bond length.
Laboratory test of Alkenes
|
Reagents |
Effect |
|
Br2/CCl4 |
Decolourise |
|
Cold dilute alkaline KMnO4 solution |
Brown ppt. of MnO2 |
|
Tollens’ reagent |
No effect |
|
Ammoniacal cuprous chloride Solution |
No effect |
Uses of Alkenes
- It is used for the production of polythene which has many applications, used in plastics.
- It is used in the production of polystyrene for use in automobile battery boxes and refrigerator parts.
- It is used in the formation of Ethane-1,2-diol which is used as an antifreeze in automobile radiators.
- It is used in the formation of propanol for acetone production.
Practice Problems
Q. 1. Will the compound given in the following reaction give β-elimination product?
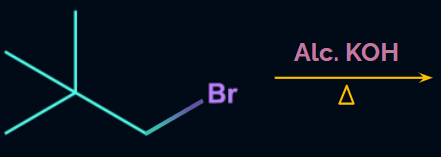
Solution:
The presence of β-hydrogen is a necessary condition for the dehydrohalogenation reaction (β-elimination reaction).
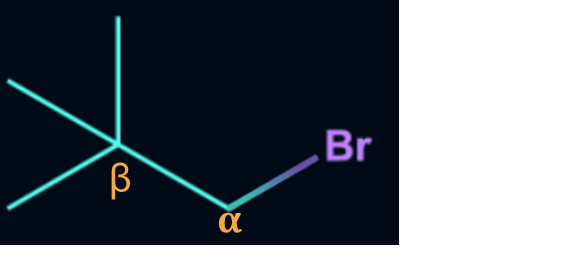
Since the given compound does not contain any β-hydrogen,
it does not undergo dehydrohalogenation reaction.
Q. 2. What is the increasing order of rate of dehydrohalogenation reaction?
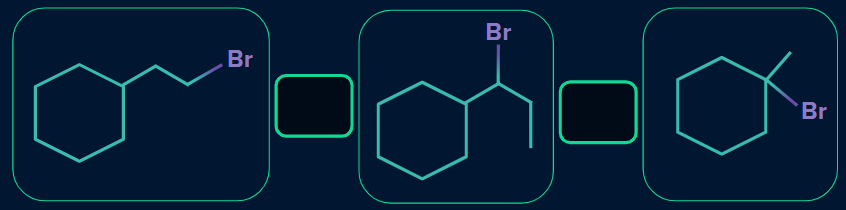
Solution:
We know that the rate of alkyl halide towards dehydrohalogenation reaction is as follows:
Tertiary alkyl halide > Secondary alkyl halide > Primary alkyl halide
(I) is the primary alkyl halide, (II) is the secondary alkyl halide, and (III) is the tertiary alkyl halide. So,
the order will be: (I) < (II) < (III).
The increasing order of rate of dehydrohalogenation reaction is: (I) < (II) < (III).
Q. 3. Find the major product of the given reaction.
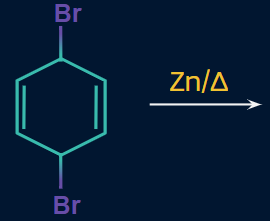
Solution:
According to the mechanism,
Zn --> Zn+2 + 2e-
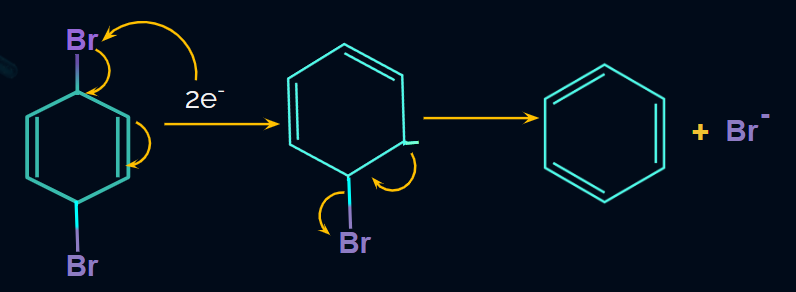
Here, delocalisation takes place and finally, another Br is eliminated, resulting in the formation of benzene as the product.
Q. 4. Find the total number of possible alkenes in the given elimination reaction.
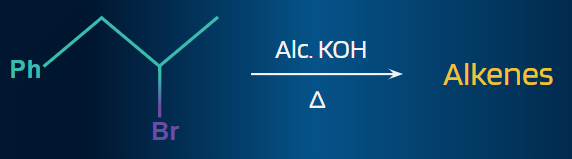
Solution:
A given reactant may include two or more structurally different groups of beta-hydrogens, in which case an E2 elimination may result in the formation of a number of constitutionally isomeric alkenes.Two alkenes are possible for the given elimination reaction, as two types of β-hydrogens are present in it.
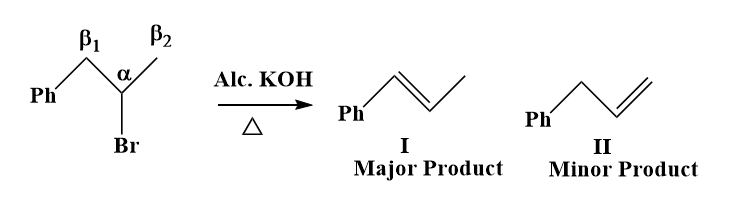
(I) is a major product, as it is more stable due to the resonance in it.
Frequently asked questions-FAQs
Q. 1. What is the general formula of alkene?
Answer: The general formula of alkene is CnH2n.
Q. 2. Give some practical uses of alkenes.
Answer: Alkene has many applications, it is used in the synthesis of plastics, alcohols, fuels, liquors, detergents.
Q. 3. Provide the pathway for the following reaction:
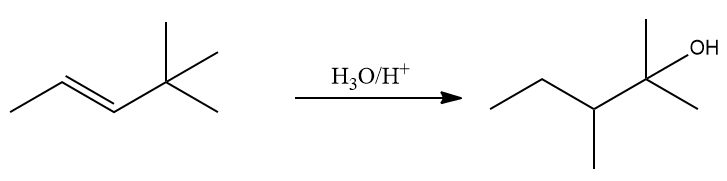
Answer: Alkenes undergo a reaction when H2O/H+ (acid catalysed hydration) is introduced and this is a Markovnikov addition.
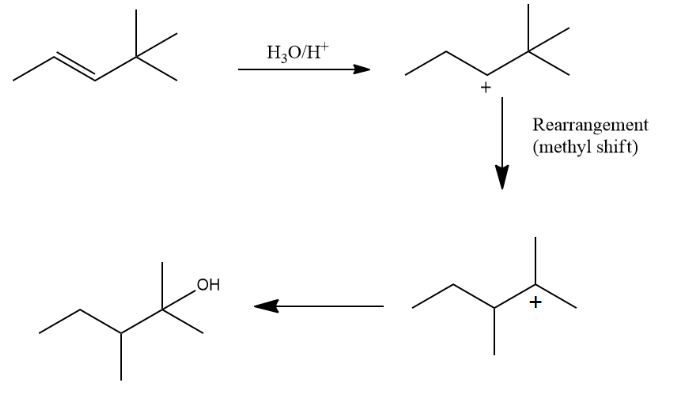
Q. 4. Is the hydrogen of alkene more acidic than alkynes?
Answer: When joined to a more electronegative atom, a hydrogen tends to be more acidic. Alkynes are sp hybridised which means its orbitals have 50 % s-character. Hence alkyne carbon atoms are more electronegative than sp2 carbon atoms of alkene. Hence alkynes are more acidic than alkenes.


