-
Call Now
1800-102-2727
Alpha particle scattering and Rutherford's atomic model-Observations, Limitations, practice problems, FAQs
Everything around is made up of atoms; ranging from a needle pin to a huge car, every object on earth is composed of atoms at the microscopic level. English Chemist and Physicist John Dalton, said that all matter is made of atoms and that they are indestructible. JJ Thomson, who also discovered cathode rays in 1897, proposed the Plum Pudding Model in order to explain the structure of an atom. He believed the atom to be a solid sphere of positive charge with electrons embedded in it like plums in a pudding. The plum pudding model is analogous to a watermelon; the seeds like a watermelon represent the negative charge while the red portion represents the positive part. But this is not true; the negative charge does not lie at the centre of an atom. Further experiments by New Zealand Physicist Ernest Rutherford in 1911 using alpha particles threw more light on the structure of an atom. In this article, we will explore Rutherford’s atomic model in detail.
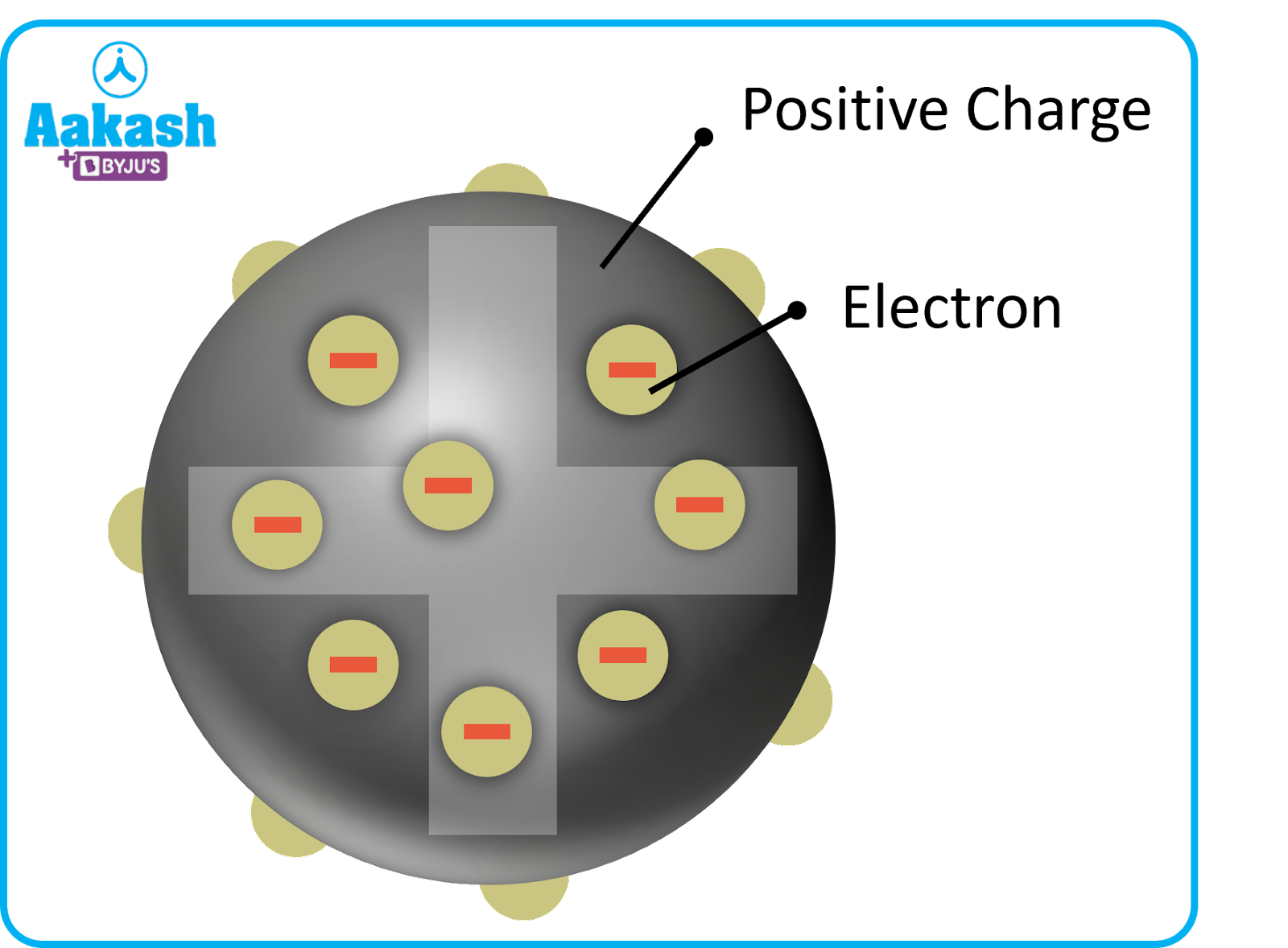
Plum Pudding model
Table of contents
- Rutherford’s alpha particle scattering experiment
- Rutherford’s atomic model
- Distance of closest approach
- Impact parameter
- Limitations of Rutherford’s model
- Practice problems
- FAQs
Rutherford’s alpha particle scattering experiment
Rutherford, along with his disciple, Geiger and Marseden, conducted an experiment in 1911, in which they projected particles (radioactive helium nuclei- from a polonium sample kept in a lead cavity. A zinc sulphide screen which is capable of showing fluorescence, is placed on the other side of the gold foil.
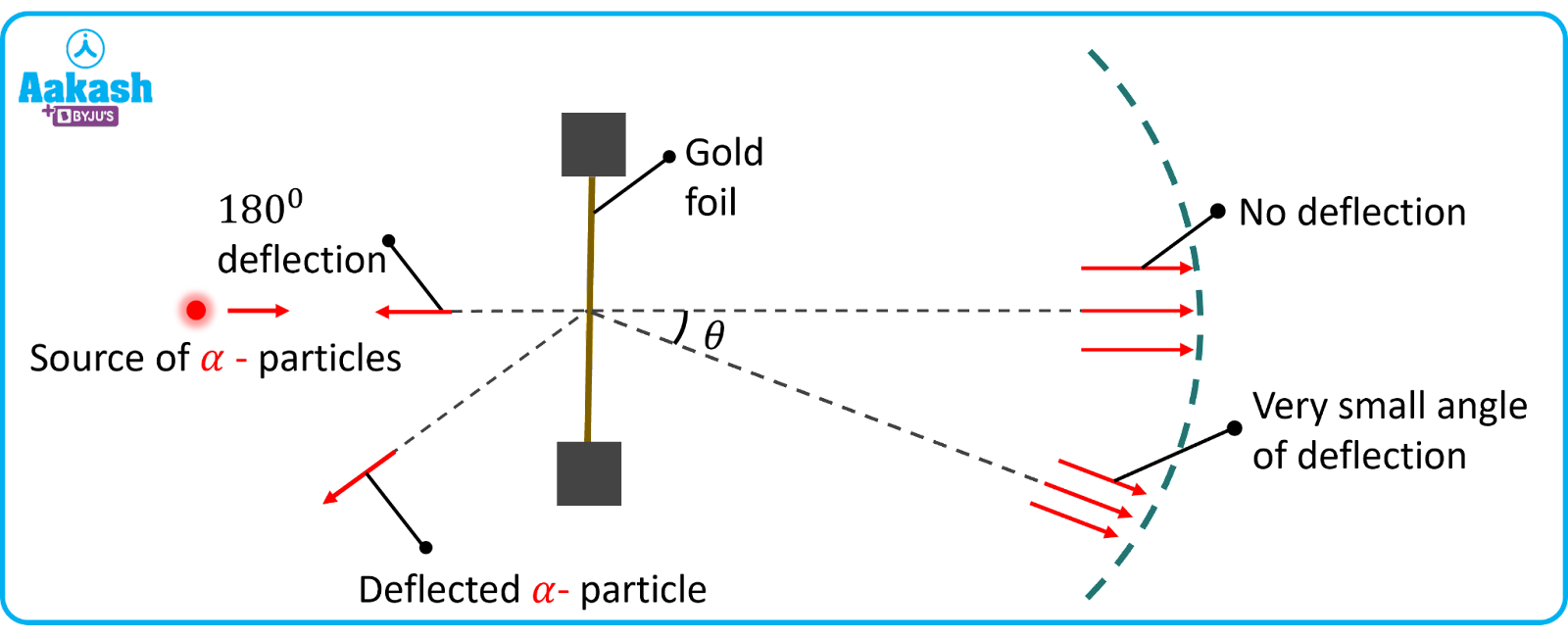
Observations
The following observations were made from the gold foil experiment:
(i) Most of the particles passed undeflected while some of them were deflected through small angles.
(ii) About 1 in 8000 particles are deflected through angles more than 900.
(iii) Very few particles were deflected through 1800. i.e they retraced their path.
(iv) The number (N) of alpha particles scattered at a scattering angle varies as
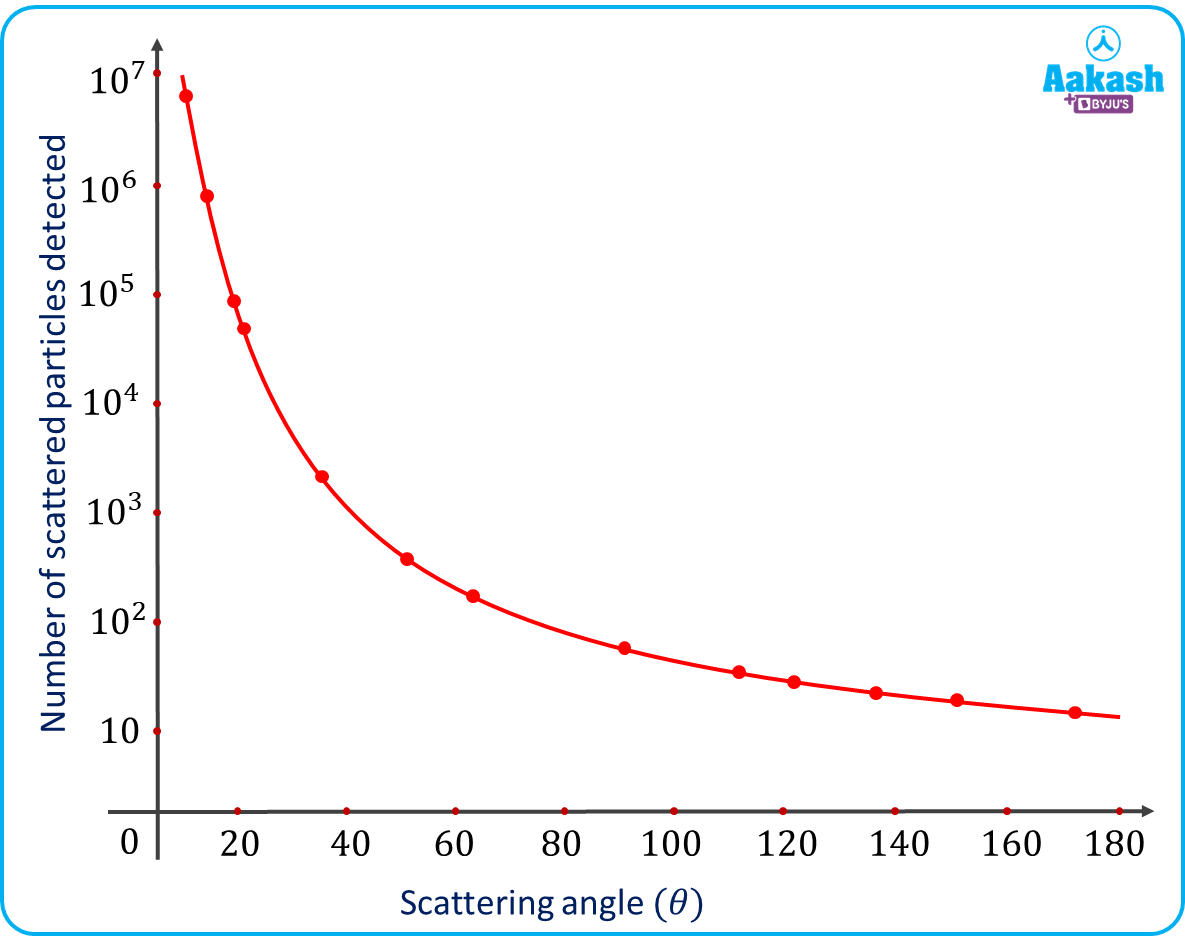
Graph showing a variation of number of alpha particles scattered vs scattering angle
Rutherford’s atomic model
From his experiment, Rutherford gave the following postulates:
(i) Most of the mass of an atom is concentrated at the centre called its nucleus.
(ii) Nucleus is positively charged and has the size of the order of 1 fermi ( 10-15 m).
(iii) Most of the space in an atom is empty; hence, most of the particles went undeflected.
Distance of closest approach
The minimum distance upto which the alpha particle can move towards the nucleus before it starts retracing its path is called the distance of closest approach (r0). When this distance is attained, almost all of its kinetic energy appears as electrostatic potential energy. If Z represents the atomic number of the nucleus, then its charge is given by Ze. An particle carries a charge of +2e. Equating kinetic energy to the electrostatic potential energy of ( particle+ nucleus) system,
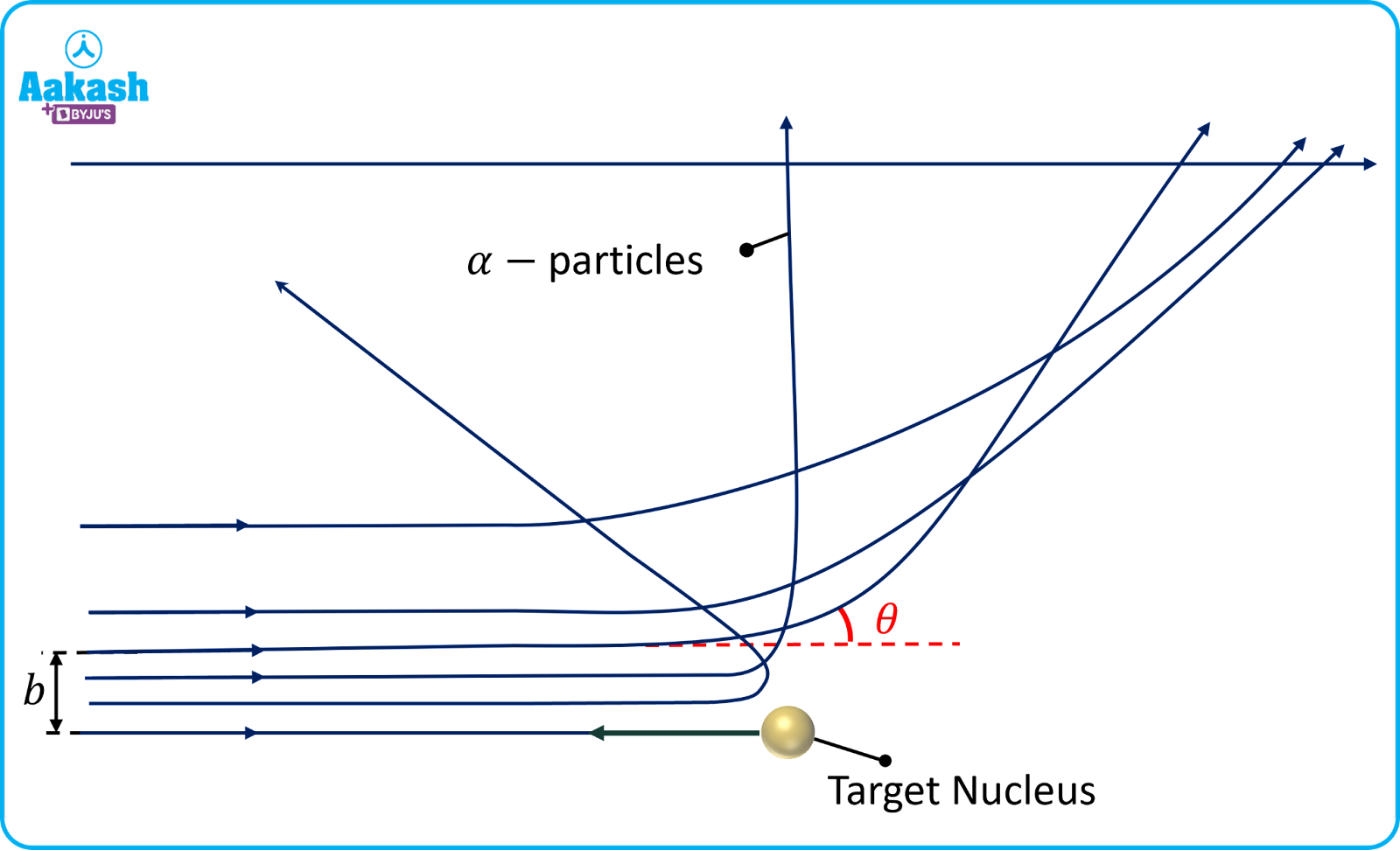
Impact parameter
The perpendicular distance from the initial velocity vector of the particle to the centre of the nucleus is called the impact parameter (b). For small values of the impact parameter, the alpha particles get scattered through a large angle. For larger values of the impact parameter, the alpha particles go nearly unscattered.
Limitations of Rutherford’s model
Rutherford’s model failed to explain the stability of an atom. When an electron is accelerated, it emits electromagnetic radiation, therefore it should lose energy, and then eventually fall into the nucleus. It also failed to explain discrete wavelengths in the hydrogen atom; As the electron spirals inwards, both its frequency of revolution and angular velocity change continuously; Hence the frequency of electromagnetic radiation emitted would change. So, atoms should emit a continuous spectrum (a spectrum having a continuum of wavelengths emitted due to electrons jumping inside an atom). But what we observe in hydrogen is only a discrete line spectrum.
Video explanation
https://www.youtube.com/watch?v=vyAVKJPS3P8&t=871s
From 11:26-20:36
Practice problems
Q1. In a Rutherford experiment, 8100 scattered particles falling per unit area per unit minute were observed at an angle of 600. Calculate how many scattered particles would fall per unit area per unit minute when the scattering angle is 1200?
(a) 800
(b) 100
(c) 600
(d) 900
Answer. d
Given
Let N be the number of alpha particles scattered; then
Q2. In a Rutherford scattering experiment, what is the correct angle of angle of scattering, when the impact parameter b=0?
(a) 600
(b) 1800
(c) 300
(d) 1200
Answer. b
We know, the impact parameter
Where, is the scattering angle
Q3. Silver has an atomic number of 47. Calculate the speed at which the protons need to be fired at a silver foil, if the particles were able to get within a distance of of the silver nucleus?
Answer. Given, distance of closest approach,
Mass of the proton,
Charge of the proton
Charge of the silver nucleus
Velocity, v=?
When the distance of closest approach is obtained, Kinetic energy= Potential energy
Q4. An particle having an energy of 5 MeV is scattered through an angle of 1800 . Calculate the distance of closest approach towards the uranium nucleus ?
(a) cm
(b)
(c)
(d)
Answer. a
Given, kinetic energy of the alpha particle=5 MeV
Charge of an alpha particle =+2e, where is the electronic charge
Atomic number of uranium, Z=92
When the distance of closest approach r0 is attained, all of the kinetic energy gets converted into electrostatic potential energy;
i.e,
Where is the kinetic energy of the alpha particle,
Solving, we get
FAQs
Q1.What was the purpose of Rutherford’s scattering experiment?
Answer. Rutherford along with Geiger and Marsden conducted the alpha particle experiment in order to study the structure of the atom. It revealed that most of the space in an atom is empty, while the positive charge is concentrated at the centre alone.
Q2.Why did Rutherford use gold foil?
Answer. Gold is a malleable material, which means it can be beaten into thin sheets. Hence, Rutherford used gold in his experiment. Making thin sheets is important as it will accommodate less atoms along the trajectory of alpha particles.
Q3.Why did Rutherford use alpha particles in his experiment?
Answer. Alpha particles have high energy upto 7.7 MeV. They travel at high speeds without losing their energy.
Q4. Where are the protons located in an atom?
Answer. The protons are located at the centre called the nucleus. Protons are high mass particles in an atom. As most alpha particles go undeflected it proves that they are not encountering any heavy particles in their path. So to infer that protons and heavy particles are at the centre of an atom takes a minute space as compared with the size of the atom.
Q5.What is the approximate size of an atom?
Answer. The size of an atom is approximately . It is measured in the units Angstrom.


