-
Call Now
1800-102-2727
Heat, Energy and Work-Definition,Examples, Practice problems,FAQs
Heat is a form of energy which is predominant in all systems. For instance, a kettle containing hot water has some heat energy. This arises due to the motion of atoms and molecules. Work is the mechanical transfer of energy from one system to another. An example of this would be the refrigerator–it transfers heat energy from a colder body to the hotter body, and in the process, takes energy input in the form of some work. Heat is capable of being converted to work. Let us consider an insulated cylinder containing a gas which is fitted with a piston on top. When heat is supplied to the container, the gas expands, pushing the piston up. In this process, work is said to be done by the system. On the other hand, if the piston is pushed down, some amount of work is said to be done on the system and temperature rises. In a nutshell, heat is capable of being converted to work. In this article, we will explore heat, energy and work in detail.
Table of contents
- Heat, energy and work
- Heat and internal energy
- First Law of thermodynamics
- Practice problems
- FAQs
Heat, energy and work
Heat is a form of energy possessed by a system. With the rise in temperature, the heat content of a system increases. The heat content of a kettle increases when it is heated further. When water freezes to form ice, heat is released. On the other hand, heat is absorbed when ice melts. This is an example of heat being released or absorbed when a phase change occurs. Heat is capable of being converted to work. When an insulated container with a gas is heated, the heat gained by the gas is used to do work. This is seen when it pushes the piston up. The heat content of a system is a path function–it depends on the path taken by the system.
The SI unit of heat, energy and work is Joule. The dimensional formula is
Note:
The sign convention for the work and heat is as follows:
Work done by the system is +ve. Work done on the system is -ve
Heat added to the system is positive and heat released by the system is negative.
Heat and internal energy
Internal energy of a gas is defined as the sum of the potential and kinetic energies of the molecules. Since, it depends upon the number of molecules (i.e., the mass) of the substance, it is an extensive property. It is a state function – it only depends on the final and initial temperature of the gas, and does not depend upon the path taken by gas.
If dU indicates the infinitesimal change in internal energy of the gas, then
dT- change in temperature, No. of moles of the gas, CV- specific heat capacity at constant volume.
For an isothermal process (temperature-constant), dT=0, hence internal change dU=0.
First Law of thermodynamics
The first law of thermodynamics is in accordance with the law of conservation of energy. Let ΔQ be the amount of heat energy supplied to a gas. Some amount of this would be used to increase the internal energy as well. The rest of the energy is used to do work ΔW. Then,
ΔQ=ΔU+ΔW--(i)
An adiabatic process is one in which no heat is lost or gained to the surroundings, i.e dQ=0.
Hence, equation (i) becomes,
ΔU=-ΔW
During compression, work done ΔW=-ve. This is because when pressure is a positive term, the integrating factor dV will be negative. ΔV=-ve in the case of compression.
During expansion, work done ΔW=+ve. ΔV=+ve in the case of expansion.
Practice problems
Q1. A certain quantity of an ideal gas is taken from state A to state B, through two different routes I and II. If Q1 and Q2 indicate the amounts of heat absorbed by the gas, then
(a) Q1=Q2 (b) Q1<Q2 (c) Q1>Q2 (d) Data insufficient.
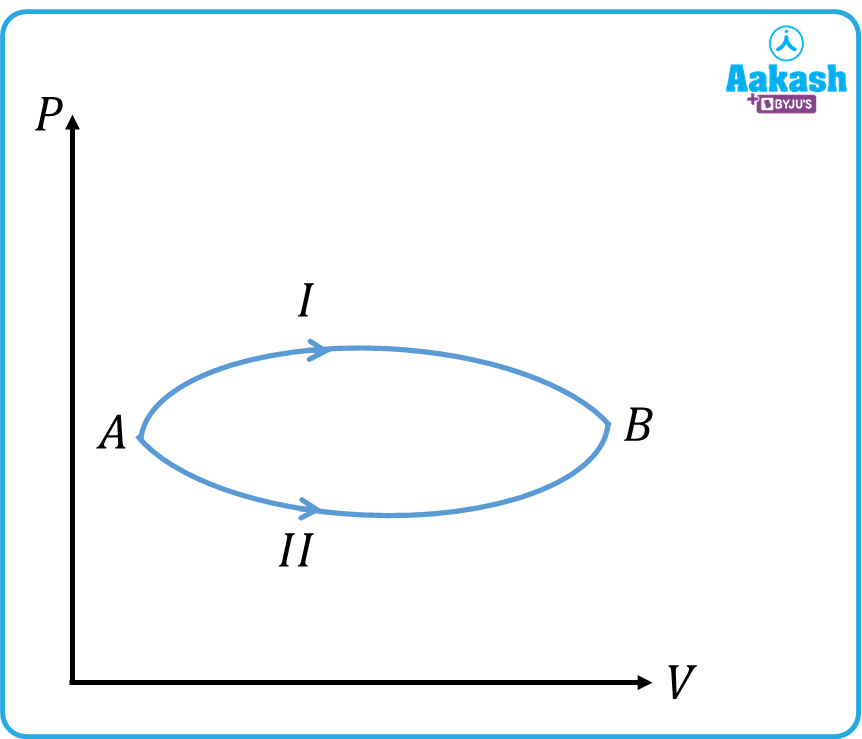
Answer. c
In the process 1,
In the process 2,
The change in internal energy is the same in both the paths.i.e . But since the area enclosed by process I with the x-axis is higher–more work is done.
Hence,
Q2. A system is taken from point a to point b, along two different paths, acb and adb as shown in the figure. In the process , the heat absorbed by the system is 60 J and 30 J work is done.
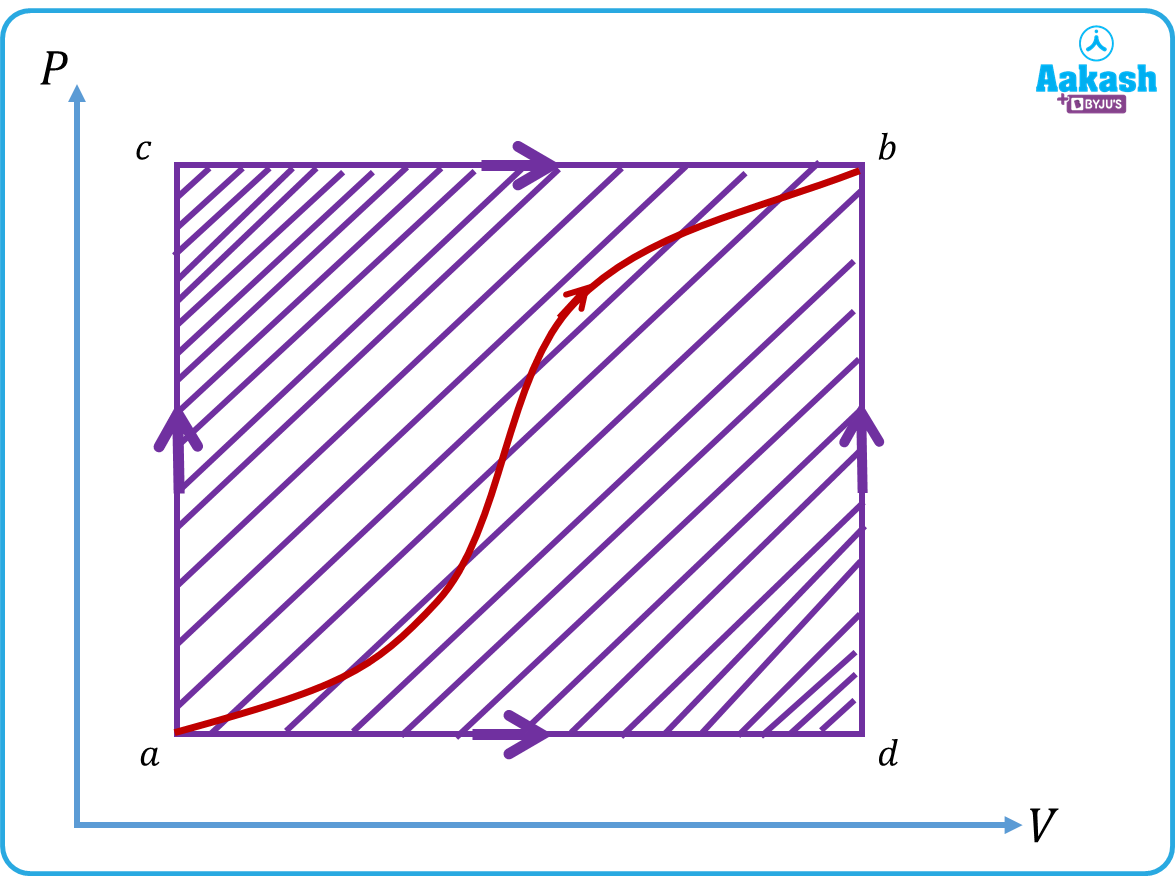
Calculate the heat flown into the system along the path if the work done is 10 J.
Answer. Given,
,
Along acb, .
. [as the change is internal energy is state dependent not path dependent quantity]
When the gas is taken along adb
Q3.In a thermodynamic process, 200 J of heat is supplied to a gas.In the process, 100 J work is done by it. Calculate the change in internal energy of the gas.
- 100 J (b) 300 J (c) 419 J (d) 24 J
A.a
Given,
Applying the first law of thermodynamics,
Q4.Given below are four processes. Identify the process for which .
(c) 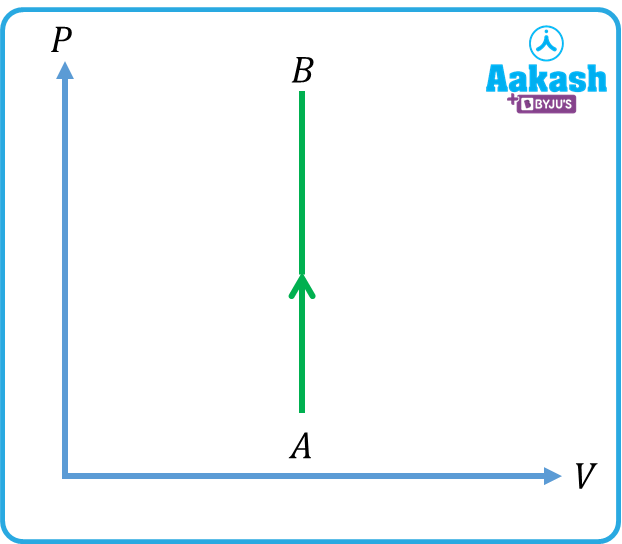
(d) 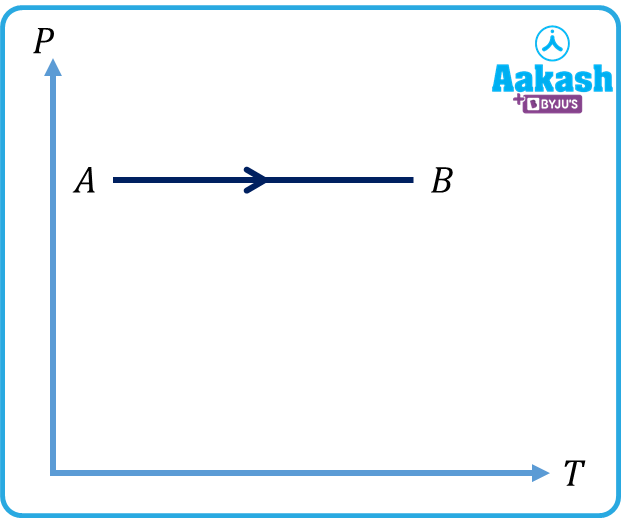
Answer. b
refers to an isothermal process. Out of the four options, (b) represents an isothermal process.
In an isothermal process, the temperature remains constant. Internal energy is a function of the temperature. So, in an isothermal process, internal energy remains the same.
FAQs
Q1. Can heat energy be used to do work?
Answer. Yes, heat energy can be converted to work. A common example of this is the heat engine, where heat is generated by the burning of fuel (coal, gas, petroleum etc). This heat is in turn used to perform work by the engine.
Q2. Does work done depend on the temperature?
Answer. If a system is cooled, heated, compressed or expanded, the change in temperature only depends upon the initial and final states, and not upon the path taken by the gas. Hence it would be appropriate to say that work done depends upon the temperature change and not the temperature of the gas.
Q3. How is heat related to power?
Answer. Power is defined as the rate of change of heat energy or work. Hence, if an engine is capable of converting more heat energy to work, it is said to be capable of delivering more power. The SI unit of heat/work is Joule, while that of power is Joule/second or Watt.
Q4. Can work be done without transferring the energy?
Answer. No. If work is done, energy is being transferred. Spring can extend/contract due to its stored elastic potential energy. According to the first law of thermodynamics, whenever work is done on the system, there should necessarily be a transfer of energy.