-
Call Now
1800-102-2727
Zero Order Integrated Rate Equation - Definition, Equation, Graphical Representation, Half Life, Practice Problems and FAQs
India is a living example of unity in diversity. There are people from different cultures, religions, castes, communities etc. but still people are united. This is one of the main reasons why our country has grown up to such an extent.

But why are we talking about unity? Well, with unity comes strength and integration is the solution of most problems.
Here, in this article as well, we are going to discuss the integrated rate equation of the zero order reaction to find out the concentration of the reactants and products at a particular instant of time.
Table of Contents
- Integrated Rate Law for Zero Order Reaction
- Unit of Rate Constant for Zero Order Reaction
- Graphical Representation of Zero Order Reaction
- Half Life of a Zero Order Reaction
- Practice Problems
- Frequently Asked Questions - FAQs
Integrated Rate Law for Zero Order Reaction
Through differential rate law we get to know how the rate of a reaction depends on the concentration of the reactants. But if we want to know the concentration of the reactants at a particular instant, it would be difficult to get using the differential rate law. This particular information can be obtained by integrating the differential rate equation between the limits.
Considering a zero order reaction;
A → B+C
As the reaction is zeroth order, the differential rate equation can be written as;
Where, R is the rate of the reaction.
d[A]dt is the change in the concentration of A with respect to time.
k is the rate constant.
The rate of the reaction does not depend on the concentration of the reactant.
Equation (i) can be written as:
⇒ d[A]=-kdt
Now, integrating the above equation under the limits [A]o to [A]t and 0 to t.
Where, [A]o is the initial concentration of A at t=0 and [A]t is the final concentration of A at time t.
⇒[A]o[A]td[A]=-k0tdt
⇒ [A][A]t[A]o=-k [A]t0
⇒ [A]t-[A]o=-k(t-0)
⇒ [A]t-[A]o=-kt....(ii)
The above equation is the Integrated rate equation of zero order reaction.
Unit of Rate Constant for Zero Order Reaction
From the integrated rate equation of zero order reaction i.e., equation (ii);
[A]t-[A]o=-kt
⇒ k=[A]o-[A]tt=mol L-1S
So, the unit of k is mol L-1s-1.
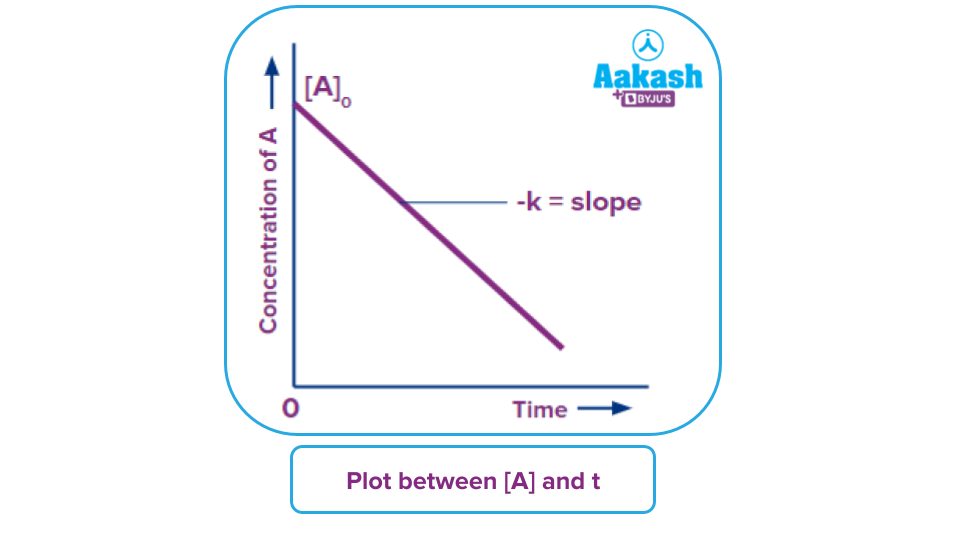
Graphical Representation of Zero Order Reaction
From the integrated rate equation of zero order reaction i.e., equation (ii);
[A]t-[A]o=-kt
⇒ [A]t=-kt+[A]o
If above equation is compared with the straight line equation, Y=mX+C.
On the Y-axis, there will be [A]t, on X-axis there will be t.
The constant slope of the straight line would be m=-k and the Y-intercept C=[A]o.
Half Life for a Zero Order Reaction
Half life (t1/2) is the time taken by a reactant to decrease its concentration by half of its initial value.
From the integrated rate equation of zero order reaction i.e., equation (ii);
[A]t-[A]o=-kt
At t=t1/2, [A]t=[A]o2.
Substituting the values in the equation, we get;
[A]o2-[A]o=-kt1/2
t1/2=[A]o2k
Practice Problems
Q. What is the time required for a zero order reaction to complete 100 % of the reaction? (Considering [A]O is the initial concentration of the reaction and k is the rate constant)
- [A]O2k
- 3[A]O2k
- 2k[A]O
- [A]Ok
Answer: (D)
The integrated rate equation for a zero order reaction is:
[A]t-[A]o=-kt
At 100 % completion of the reaction, [A]t will be zero.
So, the equation becomes;
-[A]o=-kt
⇒ t100%=[A]ok
Q. A zero order reaction has the rate constant value 0.025 M s-1. The initial concentration of the reactant A is 0.5 M. The concentration of A after 20 seconds would be:
- 0.125 M
- 0.05 M
- 0.075 M
- 0.065 M
Answer: (C)
The integrated rate equation for a zero order reaction is:
[A]t-[A]o=-kt
Putting the given values;
The integrated rate equation for a zero order reaction is:
[A]t-0.5M=-0.025 M s-1 × 17 s
[A]t=0.5M-0.425 M
[A]t=0.075 M
Q. The decomposition of ammonia gas in the presence of platinum catalysts is a zero order reaction. The concentration of ammonia when plotted against the time a straight line is obtained. The slope of the straight line is:
- 0
- 1
- k
- -k
Answer: (D)
From the integrated rate equation of zero order reaction;
[A]t-[A]o=-kt
⇒ [A]t=-kt+[A]o
If above equation is compared with the straight line equation, Y=mX+C.
On the Y-axis, there will be [A]t, on X-axis there will be t.
The constant slope of the straight line would be m=-k and the Y-intercept C=[A]o.
Q. According to the graphical representation given below for the zero order reaction.
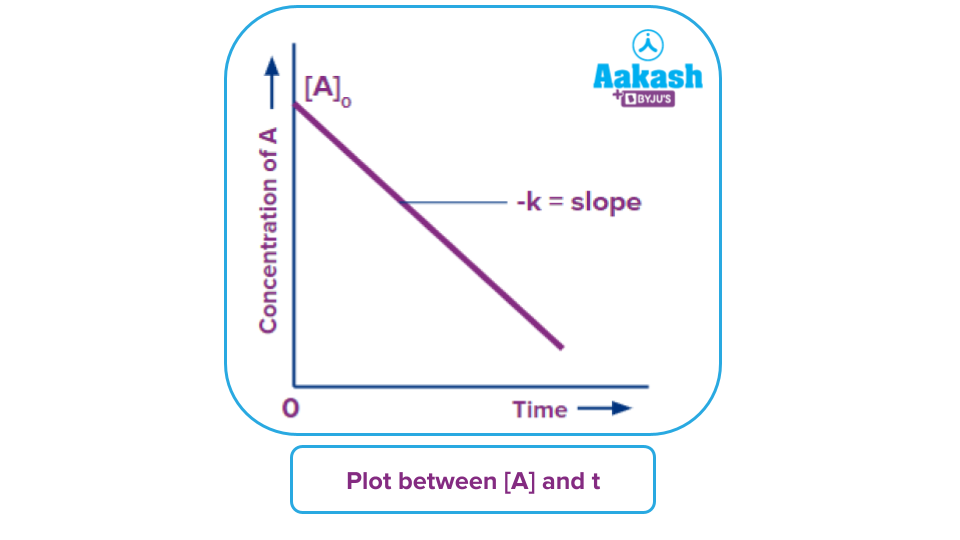
The Y- intercept of the straight line is:
- k
- [A]O
- -[A]O
- -k
Answer: (B)
From the integrated rate equation of zero order reaction;
[A]t-[A]o=-kt
⇒ [A]t=-kt+[A]o
If above equation is compared with the straight line equation, Y=mX+C.
On the Y-axis, there will be [A]t, on X-axis there will be t.
The constant slope of the straight line would be m=-k and the Y-intercept C=[A]o.
Frequently Asked Questions - FAQs
Q. What are the examples of the zero order reaction?
Answer: A few examples of zero order reactions are:
- The reaction between chlorine and hydrogen which is a photochemical reaction and it produces hydrogen chloride.
- The decomposition of nitrous oxide on a surface of platinum which is at high temperature.
- The decomposition of ammonia to produce nitrogen and hydrogen gas in presence of molybdenum catalyst.
Q. What is the order of photochemical reactions?
Answer: All photochemical reactions are zero order reactions as the rate of the reaction does not depend on the concentration of the reactants.
Q. To complete the zero order reaction, does it take infinite time?
Answer: The zero order reaction is completed in a finite time. Time taken to complete a zero order reaction can be calculated using the integrated rate equation and the value to time for 100 % completion of zero order reaction is [A]ok. Where, [A]o is the initial concentration of the reactant A and k is the rate constant.
Q. Can we say that zero order reactions are elementary?
Answer: The rate of the zero order reaction does not depend on the concentration of the reactant. But it does not mean that the zero order reaction is elementary. The zero order reaction can have various steps in the reaction mechanism.


