-
Call Now
1800-102-2727
Wilkinson’s Catalyst – Structure, Preparation, Properties, Catalytic Hydrogenation of Alkenes and Applications
Pizza’s are unanimously cherished by all. What would you feel like, if someone takes you out for a pizza treat and provides you with a pizza box? You must be thrilled at the very thought of it, won’t you?
What if, on opening this box now, you find the pizza with a missing piece?! You would probably be upset with this insufficiency of the pizza!
Now coming back to this fact in the light of Wilkinson’s catalyst, it is also a heavy-sounding complex compound (like the pizza) but is slightly incomplete in terms of valence electron count that is needed to suffice it, or make it complete (consider that to be a complete pizza!).
Wilkinson’s catalyst is a 16-electron coordination complex so it is deficient in terms of electron and stability as per the 18-electron rule. This makes it willing to fulfil its deficiency, just like the incomplete plate would cry for the missing quarters of the pizza! Hence this special metal catalyst is susceptible to binding various substrates (H2and olefins) and is thus significant for catalytic hydrogenation of alkenes.
The reaction of catalytic hydrogenation is crucial for both the synthesis of many fine compounds and bulk materials. Numerous industrial applications make use of hydrogenation. For instance, hydrogenation is employed in the petrochemical industry to change alkenes into alkanes (paraffins) and cycloalkanes. Vegetable ghee is also made from vegetable oils using this method.
Indeed this wonderful catalyst has a great ability to provide a catalytic surface for the hydrogenation of unsaturated hydrocarbons such as alkenes and alkynes. Let’s find out more about it, right here!
TABLE OF CONTENTS
- What is Wilkinson’s Catalyst?
- Wilkinson’s Catalyst – Structure
- Wilkinson’s Catalyst – Preparation
- Wilkinson’s Catalyst – Physical Properties
- Wilkinson’s Catalyst – Chemical properties
- Mechanism of Catalytic Hydrogenation of Alkene
- Selective Hydrogenation
- Wilkinson’s Catalyst – Stereochemistry
- Drawbacks of Wilkinson’s Catalyst in Partial Hydrogenation of Alkynes to Alkenes
- Applications of Catalytic Hydrogenation
- Practice Problems
- Frequently Asked Questions – FAQ
What is Wilkinson’s Catalyst?
Chlorotris(triphenylphosphine)rhodium(I), denoted by the formula [RhCl(PPh3)3], is a rhodium coordination complex and is famously known as Wilkinson's catalyst.
At room temperature, it is a solid with a reddish-brown tinge that is soluble in chlorinated solvents like dichloromethane (CH2Cl2) and tetrahydrofuran (THF), as well as hydrocarbon solvents like benzene. The substance is frequently employed in the catalytic hydrogenation of olefins, especially alkenes. Sir Geoffrey Wilkinson, a chemist and Nobel Prize winner, is credited with popularising its usage.
It is widely used in catalytic hydrogenation of alkenes. It is a homogeneous hydrogenation catalyst.
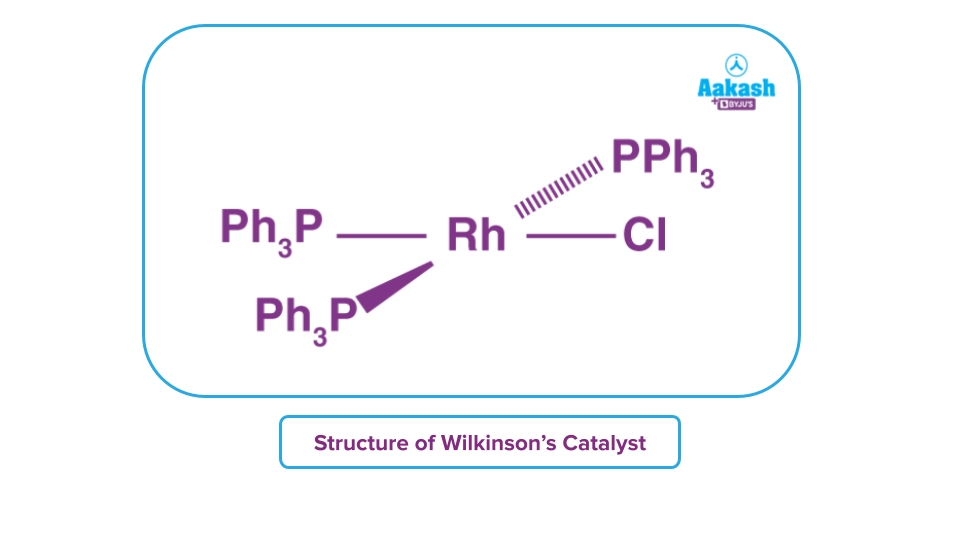
Wilkinson’s Catalyst – Structure
Single crystal X-ray diffraction shows that its structure is square planar that is slightly distorted. It is a 16-electron diamagnetic square planar complex. Rhodium in Wilkinson's catalyst is in the +1 oxidation state and exhibits dsp2 hybridization.
It is a complex of Rh(I), a d8 transition metal ion, according to the bonding analysis. According to the 18-electron rule, there are a total of 16 electrons provided by the four -PPh3 ligands (where Ph= -C6H5 group), or two electrons on each ligand. The molecule is hence vulnerable to binding substrates since it is coordinatively unsaturated (alkenes and H2).
Wilkinson’s Catalyst – Preparation
Typically, excess triphenylphosphine is added to Rhodium(III) chloride hydrates in refluxing ethanol to produce Wilkinson's catalyst. Triphenylphosphine, P(C6H5)3, a two-electron reducing agent, oxidises itself from oxidation state (+3) to (+5).
Three equivalents of triphenylphosphine (PPh3) are converted into ligands and bind themselves to the metal centre in the final product during the synthesis, whereas the fourth (PPh3) group converts rhodium (III) to rhodium (I).
In the reaction above, -PPh3 corresponds to -P(C6H5)3
Wilkinson’s Catalyst – Physical Properties
- Wilkinson's catalyst has a molar mass of 925.22 g mol-1.
- It has a melting point between 518 and 523 K.
- It cannot dissolve in water.
- It is soluble in hydrocarbon-based solvents, like benzene and tetrahydrofuran.
- The complex is diamagnetic in nature.
Wilkinson’s Catalyst – Chemical properties
- Wilkinson’s catalyst performs selective catalytic hydrogenation of unsaturated hydrocarbons (alkenes and alkynes) majorly.
- It is a complex vulnerable to binding substrates because it is coordinatively unsaturated.
- Numerous additional hydro functionalization processes, such as the hydroacylation, hydroboration, and hydrosilylation of alkenes, are also catalysed by Wilkinson's catalyst.
- Aldehyde groups, however, that are sterically unrestricted can decarboxylate, which also renders the catalyst useless.
- Since rhodium is in +1 oxidation state, hence low valent, so the metal centre can coordinate with CO strongly and perform decarbonylation of aldehydes through oxidative addition.
- This type of decarbonylation is not catalytic since the catalyst cannot be renewed. The reaction results in the formation of the very stable complex chlorocarbonylbis(triphenylphosphine) rhodium, which prevents the CO ligand from dissociating even at low temperatures.
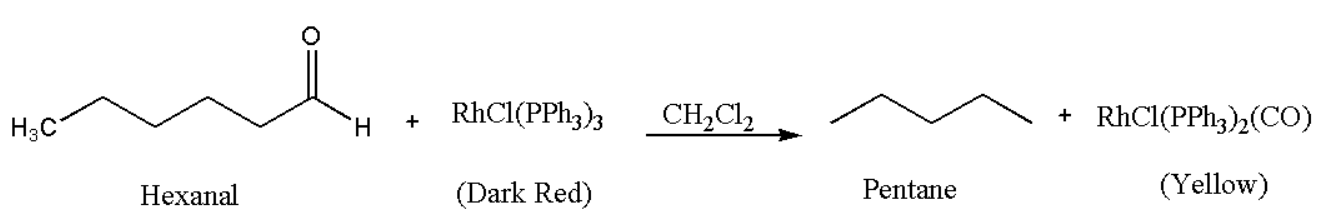
- To perform this conversion, stoichiometric quantities of the complex are needed.
- However, using DiphenylPhosphoryl Azide (DPPA), which eliminates the CO ligands from chlorocarbonylbis(triphenylphosphine) rhodium to recreate the active form of a catalyst; it is feasible to make the decarbonylation process catalytic with Wilkinson's catalyst.
- Trans-RhCl(CO) or Bis(triphenylphosphine)rhodium carbonyl chloride, is produced when carbon monoxide and RhCl(PPh3)3 combine. When aldehydes are decarbonylated, the same complex results.
- RhCl(PPh3)3 stirred in benzene solution transforms into the dimer [RhCl(PPh3)3 ]2, which is crimson in colour and is weakly soluble. This reaction highlights the lability of triphenylphosphine ligands even more.
- Wilkinson's complex changes into hydridotetrakis(triphenylphosphine)rhodium(I) complex, HRh(PPh3)4, when the base, H2, and more triphenylphosphine are present. Additionally, this complex is a functional hydrogenation catalyst, besides being an 18-electron complex.
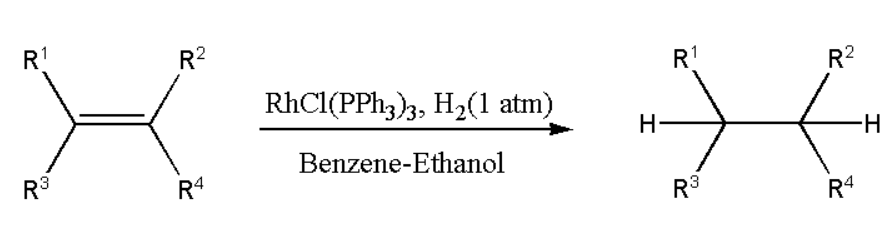
Mechanism of Catalytic Hydrogenation of Alkene
Before beginning the catalytic cycle, Wilkinson's catalyst is transformed from a pre-catalyst into an active state by shedding one triphenylphosphine ligand. The void is often filled by the solvent molecule.
- At first there is an initial dissociation of either one or two PPh3 ligand, which leads to generation of a 12-electron or 14-electron complex.
- The catalyst then produces an 18 valence electron dihydrido complex by activating the molecular hydrogen (H2) through an oxidative addition process to the metal centre.
- Rh is brought to a +3 oxidation state now. Here a π-complex is formed with the alkene. The alkene is then bound to the resulting dihydrido complex in the following step, which also results in a loss of solvent or PPh3 ligand.
- At this point, migratory insertion occurs (1,2, hydride insertion) on one of the hydrogen atoms at the double bond through intermolecular hydride insertion. This phase is the slowest and therefore known as the rate-determining step (RDS).
- As soon as the catalytic cycle is over, an irreversible reductive elimination step releases the alkane quickly. Rh is reduced to its +1 oxidation state, and the catalyst is then renewed.
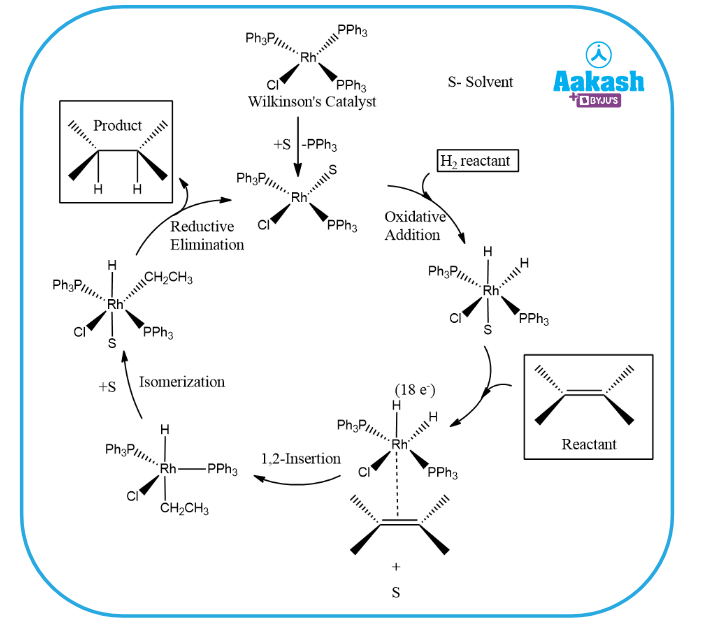

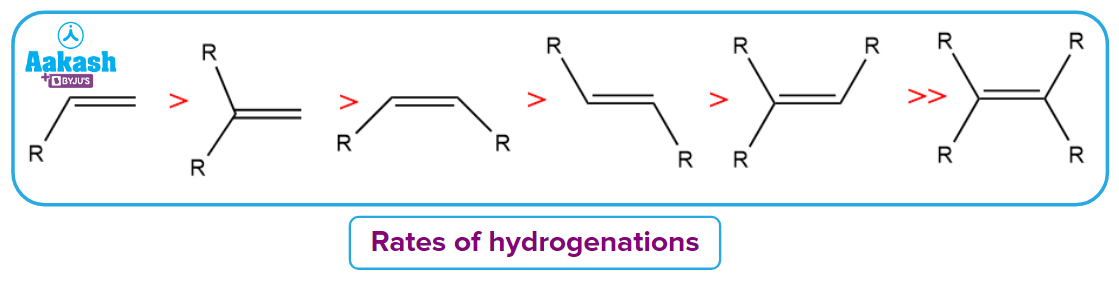
- Since the insertion into the olefin is the rate-limiting step in the process and is constrained by the high steric hindrance around the metal centre, the degree of substitution on the olefin substrate is the critical variable in terms of their hydrogenation rates.
- Terminal and disubstituted alkenes are good substrates in reality, while more hindered alkenes hydrogenate more slowly.
- The rates of hydrogenations decrease as the alkyl group substitution on double bonds increases, matching their respective metal centre binding affinities. Steric factors also have a role in its cause.
Selective Hydrogenation
Wilkinson’s catalyst is highly efficient in selective hydrogenation, which means preferential hydrogenation of certain groups present in a compound over the other.
- Selectively, endocyclic double bonds are not hydrogenated while exocyclic double bonds are hydrogenated.
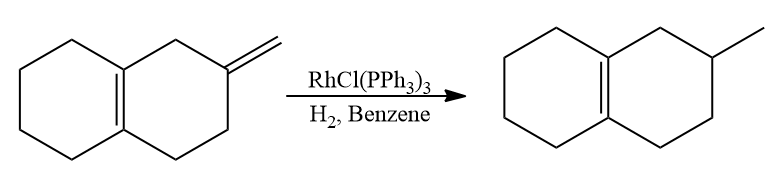
- Selective hydrogenation occurs on double bonds that are less substituted and less sterically hindered.
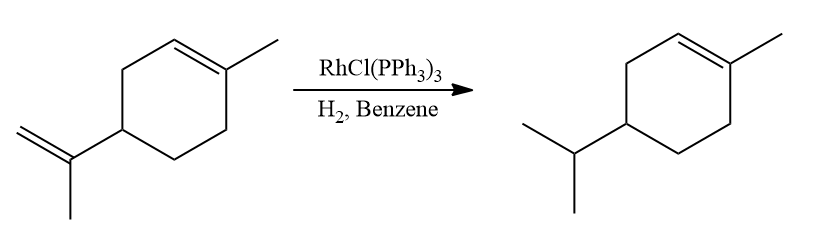
- Cis-alkenes are selectively reduced faster than trans-alkenes.
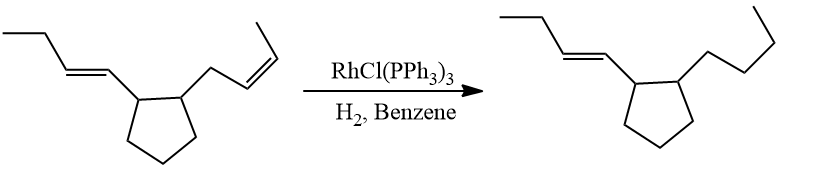
- Giving lesser priority to conjugated dienes, isolated double bonds readily hydrogenate.
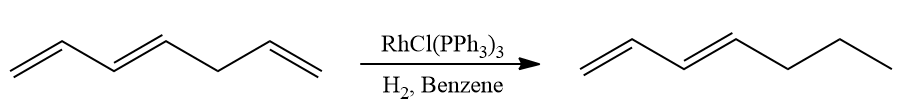
- Compared to terminal alkenes, terminal alkynes undergo hydrogenation more quickly. Use of acidic alcoholic co-solvents can improve selectivity.
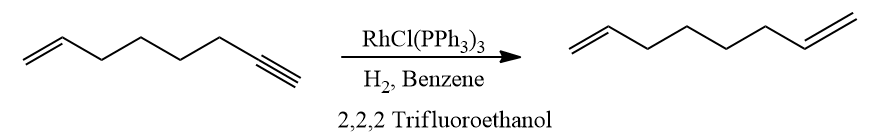
- -C=O,-C≡N, NO2, Aryl, CO2R and other functional groups are unaffected. The Wilkinson catalyst's compatibility with polar multiple bonds suggests that the metal hydride bond is largely covalent in nature.
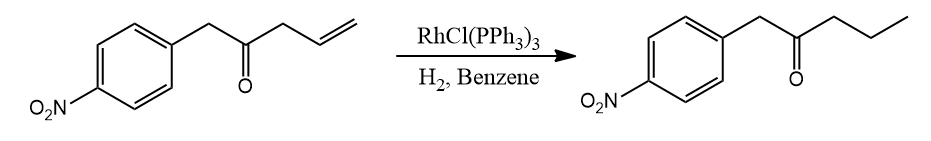
But aldehydes which are sterically unhindered, have the tendency to undergo pure decarbonylation. This is not catalytic though.
- Unsaturated substrates with polar functionality hydrogenate more quickly. It is because olefins may easily be coordinated to the catalyst with the help of the polar functional group.
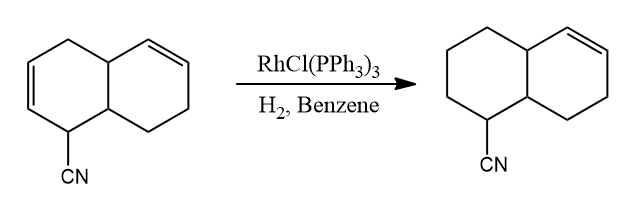
Wilkinson’s Catalyst – Stereochemistry
Stereospecific syn addition (hydrometallation) of the multiple bond is followed by stereospecific reductive elimination in hydrogenations catalysed by Wilkinson's catalyst. Therefore, alkene or alkyne hydrogenation produces syn addition products using this catalyst.
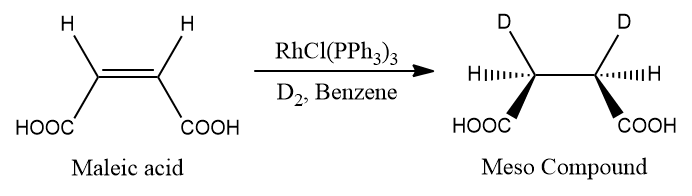
- For instance, the diastereoselective hydrogenation of maleic or fumaric acid with D2 in the presence of Wilkinson's catalyst.
- Maleic acid (cis form) is hydrogenated with D2 to produce just meso compounds.
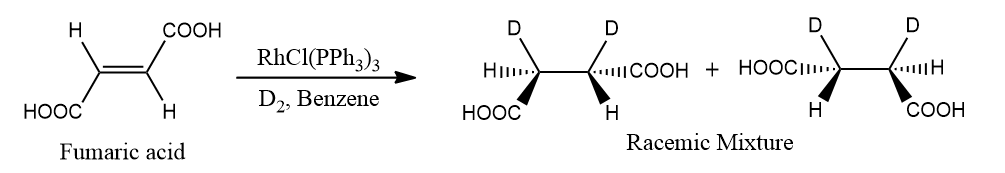
- Fumaric acid (trans form) produces a racemic mixture.
- Since Wilkinson's hydrogenation is stereospecific and stereoselective, it is a good method.
- It is stereospecific in that only one stereoisomer is produced as an exclusive good from one stereoisomer. It is because the mechanism is stereospecific.
- However, as just one diastereomer is generated as the primary product in a stereoselective manner, it is likewise stereoselective.
- Cis alkenes are the main byproducts of the hydrogenation of alkynes.
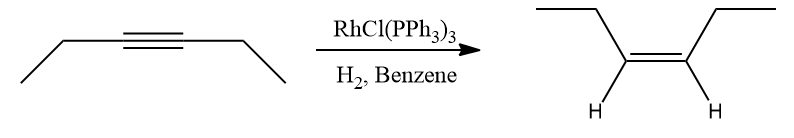
Drawbacks of Wilkinson’s Catalyst in Partial Hydrogenation of Alkynes to Alkenes
Terminal and disubstituted alkenes are good substrates in reality, while more hindered alkenes hydrogenate more slowly. Alkynes tend to be reduced to alkanes through the intermediary of the cis-alkene, making it difficult to regulate the hydrogenation of alkynes. RhCl(C2H4)(PPh3)2 is produced when ethylene combines with Wilkinson's catalyst, however it is not a substrate for hydrogenation.
Hence, the general tendency of alkynes is to undergo full saturation using Wilkinson’s catalyst as with the substrate obtained it is difficult to control and terminate the reaction.
Alkynes are more prone to additions than alkenes because of the two procurable bonds that set them apart from alkenes. These catalysts modify the placement of substituents on the newly generated alkene molecule in addition to converting them into alkenes. The catalysts either produce an anti- or a syn-addition of hydrogen, depending on which one is utilised.
Because hydrogenation involves a number of stages and is interruptible, it can be halted using modified catalysts during the transitional alkene stage, such as Lindlar's Catalyst. Lead acetate, quinoline, and palladium-calcium carbonate makeup Lindar's catalyst.
Sodium dissolved in an ammonia solvent can be used to convert alkynes to trans-alkenes. This is called the Birch reduction.
Applications of Catalytic Hydrogenation
- Alkene hydroacylation may be accomplished using Wilkinson's catalyst.
- With the aid of this coordination complex, alkenes may also undergo hydroboration and hydrosilylation.
- With the aid of Wilkinson's catalyst with hydrogen and a strong base present, functionalized tri-substituted alkenes and internal alkynes can be hydrogenated. Here, catalytically superior and highly reactive Rh(I) species are created.
- When there are many olefins present, this catalyst is quite efficient in selectively reducing the least hindered olefin.
- Hydrogenation of alkenes and alkynes via syn additions is important in various commercial processes.
- For example, alkene to alkane is used in the production of margarine, production of fatty acids etc.
Practice Problems
1. Wilkinson’s catalyst is utilised for
- Hydrohalogenation of Alkene
- Hydrogenation of Alkene
- Oxidation of Alcohols
- Dehydrogenation of alkanes
Answer: B
Solution: Wilkinson’s catalyst undergoes selective catalytic hydrogenation of unsaturated hydrocarbons (alkenes and alkynes). It is a complex that is vulnerable to binding substrates such as alkene and H2 because it is coordinatively unsaturated.
So, option B is the correct answer.
2.. What is the oxidation state of the central metal ion in Wilkinson’s catalyst?
- +3
- +2
- +1
- +5
Answer: C
Solution: Chlorotris(triphenylphosphine)rhodium(I), denoted by the formula [RhCl(PPh3)3] has the central metal ion of d8 configuration. PPh3 is a neutral ligand, Cl- has -1 charge.
Let the oxidation state of Rh be x.
So, x+(-1)+(30) = 0
So, x= +1
So, option C is the correct answer.
3. What is the stereochemistry of the products on hydrogenation of alkynes by Wilkinson catalyst?
Answer: Alkene or alkyne hydrogenation produces syn addition products using Wilkinson’s catalyst. Stereospecific syn addition (hydrometallation) of the multiple bond is followed by stereospecific reductive elimination in hydrogenations catalysed by Wilkinson's catalyst. For example, alkynes produce cis products.
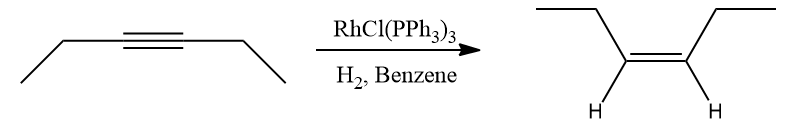
4. Alkenes undergo catalytic hydrogenation in the presence of Wilkinson’s catalyst to produce _________ product.
- Azo
- Unsaturated
- Saturated
- Aromatic
Answer: C
Solution: Alkenes on catalytic hydrogenation add hydrogen thereby forming single bonds and hence unsaturation at those points is removed. Therefore, it produces a saturated product.
So, option C is the correct answer.
Frequently Asked Questions – FAQ
1. Why is the Wilkinson catalyst, a homogeneous catalyst?
Answer: The Wilkinson catalyst is actually a reddish brown solid. It is soluble in non-polar hydrocarbons like benzene. Alkenes or alkynes can be solid, liquid or gaseous. When they are catalytically hydrogenated using Wilkinson’s catalyst, either they all are taken in solid form, or they are solubilized in non-polar and inert solvents and so the substrate and the catalyst remain in the same phase. So it is an example of homogeneous catalysis.
2. Is the Wilkinson catalyst an organometallic compound?
Answer: One of the most often used organometallic substances in organic synthesis is Wilkinson's catalyst, which is well known for homogeneously catalysing hydrogenation to convert alkenes to alkanes and alkynes to either cis-alkenes or alkanes under moderate circumstances.
3. Why is Wilkinson’s catalyst selective?
Answer: The Wilkinson's catalyst preferentially reduces (hydrogen addition) the least-hindered double bond while being unable to degrade carbon-oxygen double bonds or benzene double bonds. This makes it possible for molecules with several double bonds to specifically diminish one of them.
4. What is the geometry of Wilkinson’s catalyst?
Answer: It shows that it attains a geometry of the slightly distorted square planar structure. It is a 16-electron diamagnetic square planar complex. Rhodium in Wilkinson's catalyst is in the +1 oxidation state and exhibits dsp2 hybridization.


