-
Call Now
1800-102-2727
Victor Meyer Test: Introduction, Distinguishing Alcohols, Molecular Weight Determination, Practice Problems & Frequently Asked Questions
Have you ever met twin brothers or sisters and got embarrassed in identifying them?
Generally, everyone will have this problem and some points will be there for differentiating them, which only their close associates will be able to recognise. In the same way, in chemistry, there are many such compounds with the same formula, and functional groups that are difficult to identify, and alcohols are among them.

Likewise, in organic chemistry, there are compounds called isomers with the same molecular formula but different properties. Here again, like twins, there are isomers having almost similar chemical properties but with a small difference. Identifying or distinguishing such compounds is a difficult task.
One such compound is alcohol. With the same formula, there can be three types of alcohol-namely primary, secondary and tertiary. Victor Meyer test helps differentiate primary, secondary, and tertiary alcohols.
Victor Meyer’s test is also useful in determining the molecular weight of compounds.
In this article, we will discuss in detail the Victor Meyer test.
Table of Contents:
- Introduction to Victor Meyer Test
- Distinguishing Alcohols by Victor Meyer Test
- Determination of Molecular Weight and Vapour Density by Victor Meyer Test
- Practice Problems
- Frequently Asked Questions(FAQs)
Introduction to Victor Meyer Test:
German chemist Victor Meyer made substantial contributions to both inorganic and organic chemistry. His discoveries of nitrolic acids, thiophene, and the Victor Meyer Apparatus for measuring vapour densities are only a few of his most well-known works.
Victor Meyer also developed the test that differentiates primary, secondary, and tertiary alcohol. One of the often employed techniques to distinguish between primary (1°), secondary (2°), and tertiary (3°) alcohols are the Victor Meyer Test. In Victor Meyer test, alcohol (1°, 2°, and 3°) has a distinctive reaction solution colour that allows for easy differentiation.
Let us first see what primary alcohol, secondary alcohol and tertiary alcohol are.
Alcohols are organic compounds containing a substituent ‘- OH’ group attached to the carbon. It is classified into three groups: primary alcohol, secondary alcohol, and tertiary alcohol depending on how many more carbon atoms are directly linked to the carbon that contains the hydroxyl group (-OH).
Primary alcohols are the alcohols where the carbon atom of the hydroxyl group (-OH) is connected to only one single alkyl group.
Secondary alcohols are the alcohols where the carbon atom of the hydroxyl group (-OH) is connected to two alkyl groups.
Tertiary alcohols are the alcohols where the carbon atom of the hydroxyl group (-OH) is connected to three alkyl group
A) Distinguishing Alcohols By Victor Meyer Test
a) Experimentation:
The Victor Meyer test uses the colour of the solutions that each type of alcohol produces following a sequence of reactions to distinguish between primary, secondary, and tertiary alcohols.
Step-1: Heat the given alcohol with a small amount of phosphorous and iodine or hydrogen iodide.
Step 2: Treat the product with silver nitrite solution.
Step-3: Add freshly prepared nitrous acid (by mixing sodium nitrite crystal with dilute hydrochloric acid)
Step-4: Add sodium or potassium hydroxide aqueous solution to make the solution alkaline.
Observation: Red colour indicates the presence of primary alcohol.
Blue colour indicates the presence of secondary alcohol.
No colour indicates the presence of tertiary alcohol.
b) Mechanism of Distinguishing Alcohol by Victor Meyer Test:
All primary, secondary and tertiary alcohols get converted into iodoalkanes and then to nitroalkanes on treatment with P & iodine mixture and with silver nitrite respectively.
Both primary and secondary alcohols differ in their reactions with nitrous acid, producing different products that develop red or blue colour in an alkaline medium respectively. Tertiary alcohol does not react with nitrous acid and hence is colourless on the addition of potassium hydroxide.
Primary alcohols:
Primary alcohols on reaction with Victor Meyer reagents gives red colour. The following is the reaction’s mechanism
Step-1: Primary alcohol on reaction with concentrated hydrogen iodide (HI) or red phosphorus and iodine to give iodoalkane.
Step-2: This iodoalkane is converted to give nitroalkane, on treatment with silver nitrite.
Step-3: This nitroalkane on treatment with nitrous acid forms nitrolic acid.
Step-4: This nitrolic acid produces the red colour in an alkaline medium
Secondary alcohols:
Secondary alcohols on reaction with Victor Meyer reagents gives Blue colour. The following is the reaction’s mechanism
Step-1: Secondary alcohol on reaction with concentrated hydrogen iodide (HI) or red phosphorus and iodine to give iodoalkane.
R2-CH-OH + (Red P+I2) or Conc HI → R2-CH-I
Step-2: This iodoalkane which is formed is treated with silver nitrite to give nitroalkane.
R2-CH-I+ AgNO2 → R2-CH-NO2
Step-3: This nitroalkane on treatment with nitrous acid forms pseudo nitrol.
R2-CH-NO2 + HNO2→ R2- C(N=O)-NO2
Step-4: This pseudo nitrol on reaction with an alkali like potassium hydroxide (KOH) gives blue colour
Tertiary alcohols:
Tertiary alcohols on reaction with Victor Meyer reagents gives a colourless solution. The following is the reaction’s mechanism
Step-1: Tertiary alcohol on reaction with concentrated hydrogen iodide (HI) or red phosphorus and iodine to give iodoalkane.
R3-C-OH + (Red P+I2) or Conc HI → R3-C-I
Step-2: This iodoalkane which is formed is treated with silver nitrite to give nitroalkane.
R3-C-I+ AgNO2 → R3-C-NO2
Step-3: When this nitroalkane is treated with nitrous acid, there is no reaction because of the lack of hydrogen on the carbon atom.
R3-C-NO2 + HNO2 → No reaction(colourless)
B) Determination of Molecular Weight and Vapour Density by Victor Meyer Test:
a) Experimental setup:
The experimental setup contains a Victor Meyer tube. It is a long glass tube with a bulb-like structure at the bottom. It has a wide opening at the top to introduce the sample and a connection to a delivery tube. The tube is placed in a vessel containing a liquid that has a boiling point of at least 30° C about that of the sample. The delivery tube is connected to a bee-hive shelf filled with water.
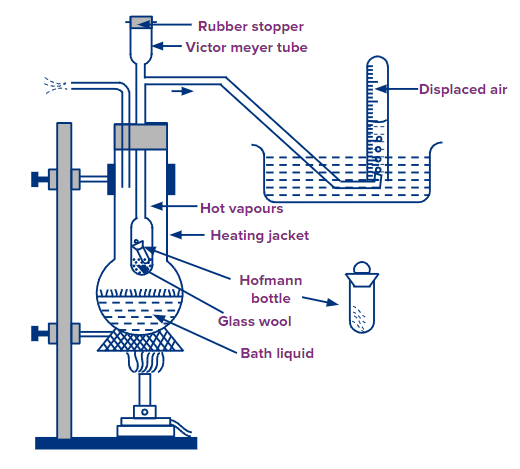
b) Experimentation:
The liquid in the outer vessel is heated to expel all the air present in the Victor Meyer and delivery tube. A graduated cylinder filled with water is placed inverted on the bee-hive self. A sample tube containing a known amount of the sample is quickly dropped inside the Victor Meyer tube containing asbestos at the button so as not to break. The sample immediately vaporises and expels an equal amount of air into the graduated cylinder that pushes the water down. The volume of the air in the graduated cylinder is noted. The volume is corrected to the volume at STP.
c) Calculation:
Let the volume of vapours at STP be V mL.
One mole of any compound occupies 22400 mL volume at STP.
22400 mL of vapours are obtained from 1 mol of the compound.
V mL of vapours are obtained from (V22400) mol of the compound
Number of moles of the substance present in the known weight = WMW
Therefore,
Molecular weight =MW =
Since Molecular weight = 2 X vapour density,
Vapour density of the sample =
d) Limitations
This method is applicable only to
- volatile solids and liquids,
- that do not decompose at their vaporisation temperature and
- that do not react or dissolve in water.
- Manual mistakes can cause big errors in the determination.
Practice Problems:
Q1. Which of the following components is not formed when secondary alcohols are tested using the Victor Meyer technique?
(A) Nitrolic acid
(B) Iodoalkane
(C) Nitroalkane
(D) Pseudo Nitrol
Answer: (A)
Solution: When testing for primary alcohol, nitrolic acid is produced; it is not produced when testing for secondary alcohol.
Q2. Alcohol that results in a colourless solution in the Victor Meyer test is:
(A) CH3 CH2 OH
(B) (CH3)2CH-OH
(C) (CH3)3C-OH
(D) All of these
Answer: (C)
Solution: The three types of alcohol listed are primary alcohol (CH3 CH2 OH), secondary alcohol
{(CH3)2CH-OH }and tertiary alcohol {(CH3)3C-OH}. In the Victor Meyer experiment, primary alcohols produce a red colour, secondary alcohols a blue colour, and tertiary alcohols a colourless solution.
Q3. Acetaldehyde (Ethanal) fails which of the following tests?
(A) Victor Meyer test
(B) Iodoform test
(C) Tollens’ test
(D) Fehling's test
Answer: (A)
Solution: Ethanal, which falls under the category of an aldehyde, cannot give a positive Victor Meyer test. Only low molecular weight primary, secondary, and tertiary alcohols can give a positive Victor Meyer test.
Q4. A + HNO2 + NaNO2 → Blue colour solution. What is A in this reaction?
(A) CH3 CH2 OH
(B) (CH3)2CH-OH
(C) (CH3)3C-OH
(D) All of these
Answer: (B)
Solution: As the Victor Meyer test's final step, the given reaction demonstrates that A is secondary alcohol because secondary alcohol will form blue colour in Victor Meyer test. So the secondary alcohol in the given options is (CH3)2CH-OH.
Frequently Asked Questions(FAQs):
Q1. Why doesn't phenol give a positive Victor Meyer test?
Answer: The first stage of the test is to create iodoalkanes using a concentrated hydrogen iodide (HI) or (Red P + I2) mixture, but this is not possible with phenols since the reaction mechanism of the reaction breaks their aromaticity and makes them unstable. The phenol molecule's partial double between carbon and oxygen, which makes it stronger than typical molecules, is the other factor.
Q2. How is nitrous acid incorporated into the reaction?
Answer: As a combination of a NaNO2 and HCl, nitrous acid is incorporated into the procedure in this test. Its unstable nature is the main reason for adding nitrous acid in this way.
Q3. Why is the tertiary alcohol final solution colourless in the Victor Meyer test?
Answer: In this test, the final tertiary alcohol solution is colourless because it fails to react with the nitrous acid in the previous step since it lacks a hydrogen atom to displace in the reaction.
Q4. Does the Victor Meyer test show ethanol or ethyl alcohol (CH3CH2OH)?
Answer: Yes, ethanol (CH3CH2OH) being primary alcohol shows a positive Victor Meyer test and gives red colouration.


