-
Call Now
1800-102-2727
Vacuum Distillation - Distillation, Their types, Vacuum distillation, Practice Problems and FAQs
We've all probably heard of the essences utilized in many foods and beverages, such as the orange flavor found in orange juice.
However, the orange juice doesn't actually contain any fresh oranges. It is entirely composed of orange essence.

Are you curious about the process used to extract this orange essence?
Vacuum distillation is a crucial step in the extraction of plant essences for the manufacturing of beverages and foods. It is also employed in the chemical and pharmaceutical sectors.
Let's discuss in detail what is Vacuum distillation!
Table of Contents
- Distillation
- The function of Raoult's Law and Dalton's Law
- Types of Distillations
- Vacuum Distillation
- Practice Problems
- Frequently Asked Questions
Distillation:
Distillation is the process of selectively boiling a component of liquid in a mixture and then condensing it thereafter. It is a method of separation that can be applied to either get more of one particular component out of a mixture or separate it out almost completely. By putting one of the liquid mixture's components into a gaseous state, the distillation process takes advantage of the difference in boiling points of the different liquids.
It is significant to highlight that it is a physical separation rather than a chemical reaction.
The function of Raoult's Law and Dalton's Law:
The boiling point of a liquid is the temperature at which its vapor pressure reaches equilibrium with the atmospheric pressure. The formation of gas bubbles at the bulk of the liquid causes it to change from its liquid state to its vapor state at this temperature.
It is significant to remember that a liquid's boiling point varies depending on the atmospheric pressure. Water, for example, has a boiling point of 100oC at sea level but a boiling temperature of 93.4o C at an altitude of 1905 m.
The distillation procedure depends on both Dalton's law and Raoult's law for a mixture of liquids. Raoult's law states that the mole fraction of a pure liquid component multiplied by its vapor pressure gives the partial pressure of that pure liquid component in an ideal liquid mixture. The overall pressure exerted by a gaseous mixture is equal to the total of partial pressures of all the component gasses, according to Dalton's equation of partial pressures.
When a liquid mixture is heated, the vapor pressure of each component rises, raising the combined vapor pressure as a result.
Why Is Complete Purification of a Mixture by Distillation Impossible?
All the volatile components of a mixture of liquids boil when the mixture reaches its boiling point. The amount of an element in the resulting vapor is determined by the contribution of the component to the mixture's overall vapor pressure, though. Because of this, compounds with greater partial pressures can condense in vapors whereas those with lower partial pressures can condense in liquids. But there is a range of boiling points of any liquid and that range may overlap with other liquids. Hence, it is not possible to get a complete or pure component from a liquid mixture.
Types of Distillations:
There are many types of distillation and given below as:
- Simple distillation
- Fractional distillation
- Vacuum distillation
- Zone distillation
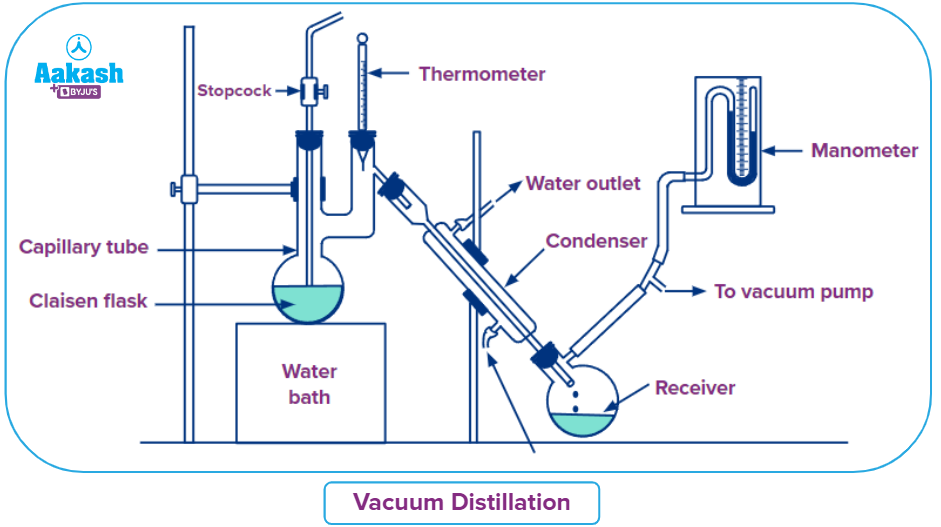
Vacuum Distillation:
For separating mixtures of liquids with extremely high boiling points, vacuum distillation is the best option. Heating to high temperatures is an ineffective way to get these chemicals to boil. As a result, the environment's pressure is reduced.
The component can boil at lower temperatures due to the reduction in pressure by using a vacuum pump. The component becomes a vapor once its vapor pressure reaches the ambient pressure. The distillate is then formed when these fumes are condensed. In order to get highly pure samples of chemicals that break down at high temperatures, vacuum distillation is also performed.
Related Video Link: Types of Distillation | CHEMISTRY | JEE | Concept of the Day | SM Sir
Practice Problems:
Q1. What is the basic principle on which the distillation process is carried out?
- Difference in the mass of components of mixture
- Difference in the temperature of components of mixture
- Difference in the volume of components of mixture
- Difference in the density of components of mixture
Answer: (B)
Solution: The basic principle on which the distillation process is carried out is a difference in the temperature of components of the mixture. When a mixture is heated, the component having a lower boiling point will evaporate fast and can be condensed in a different container while other components remain in the mixture.
Hence, option (B) is the correct answer.
Q2. What are two processes involved in the distillation process?
- Evaporation and Sublimation
- Evaporation and Condensation
- Evaporation and Desublimation
- Condensation and Sublimation
Answer: (B)
Solution: Distillation is based on the fundamental idea that different components of a mixture have different temperatures to one another. The component with the lowest boiling point will quickly evaporate when a mixture is heated and may condense in another container while the other components stay in the mixture. Hence, evaporation and condensation are the two different processes involved in distillation.
Hence, option (B) is the correct answer.
Q3. Which of the following laws are used in the distillation process?
- Dalton’s Law
- Raoult’s law
- Both (A) and (B)
- None of these
Answer: (C)
Solution: For a mixture of liquids, the distillation process is dependent on both Dalton's law and Raoult's law. According to Raoult's law, the partial pressure of a pure liquid component in an ideal liquid mixture is equal to the mole fraction of the pure liquid component times the vapor pressure of the pure liquid component. According to Dalton's equation of partial pressures, the total pressure a gas mixture exerts is equal to the sum of the partial pressures of its individual gasses.
When a liquid mixture is heated, the vapor pressure of each liquid increases, which raises the combined vapor pressure.
Hence, option (C) is the correct answer.
Q4. What happens in Vacuum distillation, when the vacuum pump is attached to the apparatus?
- By utilizing a vacuum pump, the component can boil at higher temperatures due to the decrease in pressure.
- By utilizing a vacuum pump, the component can boil at lower temperatures due to the increase in pressure.
- By utilizing a vacuum pump, the component can boil at higher temperatures due to the increase in pressure.
- By utilizing a vacuum pump, the component can boil at lower temperatures due to the decrease in pressure.
Answer: (D)
Solution: Trying to get these substances to boil at high temperatures is pointless. The pressure on the environment is thereby decreased.
By utilizing a vacuum pump, the component can boil at lower temperatures due to the decrease in pressure. When a component's vapor pressure approaches ambient pressure, it turns into a vapor.
Hence, option (D) is the correct answer.
Frequently Asked Questions-FAQs:
1. What is Simple Distillation?
Answer: Simple distillation comprises rapidly condensing the vapor after heating a liquid mixture to the boiling point. It is used for liquids whose boiling points differ significantly. (at least 25o C). Raoult's law controls a liquid's purity.
2. Describe Air-Sensitive Vacuum Distillation.
Answer: Vacuum distillation is used to separate substances that rapidly react with air and are sensitive to it. This method is frequently known as air-sensitive vacuum distillation.
3. What are some of the important purposes of distillation?
Answer: Some Important applications of Distillation process are
1. Many methods of purifying water rely heavily on distillation. Lead-acid batteries and small-volume humidifiers are only two examples of the many uses for distilled water.
2. This technique is used to purify a variety of fermented foods, including alcoholic beverages.
3. By lowering the vapor pressure of the crude oil, oil stabilization is a crucial sort of distillation that makes safe storage and transportation possible.
4. By using the cryogenic distillation method, it is possible to split air into nitrogen, oxygen, and argon.
4. Why is fractional distillation done using plastic beads?
Answer: The surface area is increased by using steel wool or plastic beads, which enhances the separation between the liquids being distilled.


