-
Call Now
1800-102-2727
Tautomerism: Tautomerism, Types, Condition for Tautomerism, Aromaticity, Factors affecting Percentage of Enol Content, Practice Problems and FAQs
Have you ever played see-saw with your friends?
Well, I believe most of us have played this during our childhood.
Imagine you and your best friend went to a park and decide to play see-saw to revive some childhood memories.

I guess you must be feeling nostalgic. The mechanism of see saw is very simple to understand, the heavier mass will remain at the bottom and the lighter mass will go up. You may have have noticed that irrespective of the weight of the person sitting on the see-saw, the one who use more force remains at the bottom and the other will go up.
Similar phenomena occurs in some organic compounds. Some organic compounds having same molecular exist in two forms which are in dynamic equilibrium. This mechanism is called tautomerism. Let’s understand the concept of tautomerism, mechanism and types.
Table of content
- Tautomerism
- Types of tautomerism
- Condition for tautomerism
- Aromaticity
- Factors affecting Percentage of Enol content
- Practice problems
- Frequently asked question?
Tautomerism
This is a kind of isomerism where two different structures of the same molecular formula but of different functional group in equilibrium with each other due to the oscillation of a monovalent acidic atom (hydrogen) between polyvalent atoms like O and N
Oscillation is a to and fro motion. Here to fro motion of hydrogen will occur. An alpha hydrogen leaves and joins another electronegative atom at third in this process. This is called as migration of hydrogen. 1,3 H- migration will occur. Tautomerism is also called as dynamic isomerism due to this phenomena.
Types of tautomerism:
Let’s see ftwo examples to understand clearly:
- Keto-enol tautomerism
- Nitro group to pseudo nitro
Keto-enol tautomerism
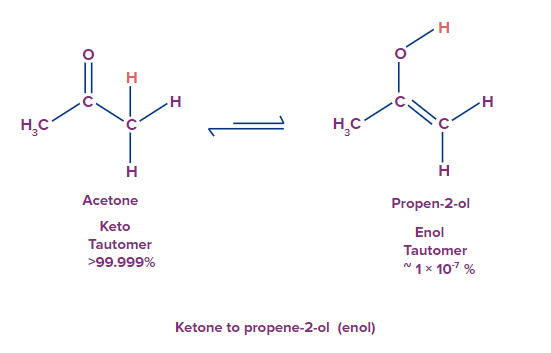
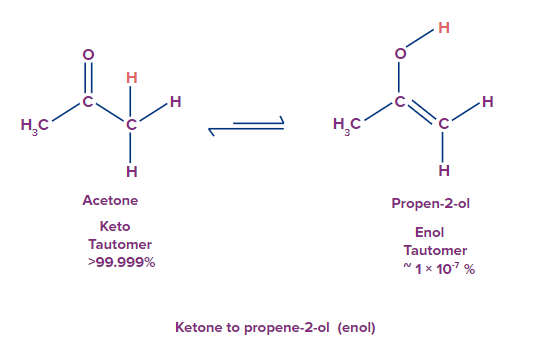
ketone to prop-ene-ol (enol)
This is an example of keto-enol isomerism.
Oscillation of hydrogen occurs from 1st position (-CH3) to third position where oxygen atom is present. This oxygen atom acquires hydrogen atom from the methyl group and becomes (-OH). As hydrogen atom is transferred from first position to third position, the double bond between carbon and oxygen shifts in between the first and 2second carbon atom leaving a lone pair of electrons on the oxygen atom. By this method acetone exists in dynamic equilibrium with propen-2-ol.
If you look closely at this equilibrium then you will find that both have the same molecular formula that is C3H6O but both have different functional group. As this equilibrium is established between ketone and enol group, hence sometimes it is called as keto-enol tautomerism.
Nitro group to pseudo nitro tautomerism
Tautomerism is not confined to only keto-enol forms. It can be shown by other compounds too. Let’s discuss some of the examples
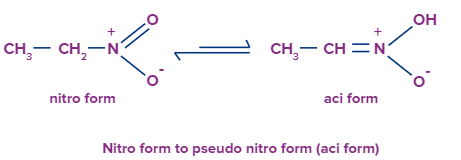
In this equilibrium it can be observed clearly that the migration of hydrogen is taking place between 1st position carbon atom to the oxygen atom attached to nitrogen atom. Just as the previous one hydrogen atom from methyl group will leave its position to combine with oxygen atom. Double bond will be shifted to the neighbor carbon atom and a lone pair of electron will be there at oxygen atom.
Example of imine group to enamine.
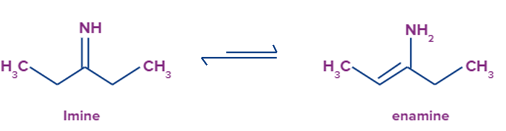
If there’s a double bond between carbon atom and nitrogen atom in an organic compound then it is called as imine group whereas if there is a double bond between two carbon atoms in an organic compound which contains nitrogen atom then it is called as enamine. Migration of hydrogen atom leads to formation of dynamic equilibrium between these two compounds.
Till now we have understood that how the migration of hydrogen occurs between these compounds. Now the question comes:
Which compounds show tautomerism?
Equilibrium means, products will form at both side then which product will be formed majorly?
Let’s try to find out the answer of first question
Condition for tautomerism
As we discussed earlier lot of people make this mistake of considering tautomerism as keto-enol tautomerism. No, tautomerism is shown by lot of different compounds. Out of which we discussed few in the above section. There’s a special condition for a compound to show tautomerism.
The compound must have a structure discussed below
X-Y=Z
X= any atom attached to hydrogen but remember one thing it should not be hydrogen only.
Y= any atom which can form double bond
And Z= It should be either carbon, oxygen, nitrogen or sulphur
Let’s check few examples whether a compound will show tautomerism or not
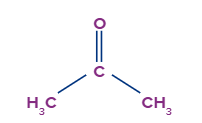
Acetone, the structure of ketone have X-Y=Zand satisfies the condition for tautomerism.
Here X= CH3, Y= -C- and Z= O atom.
Don’t consider alpha hydrogen it will complicate.
Pseudo nitro - nitro .
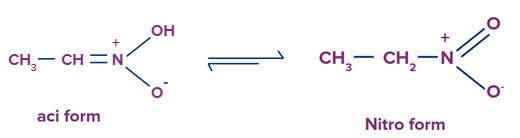
Here, you must have taken methyl group as X and oxygen atom as Z. well if you did it then its wrong. Here X= -OH ( as discussed early it should have one hydrogen atom doesn’t necessarily mean only alkyl group), Y= nitrogen atom and Z= -CH. it also satisfies the condition for tautomerism.
In case of Pivaldehyde, X does not have hydrogen atom. Hence, this compound won’t show tautomerism.
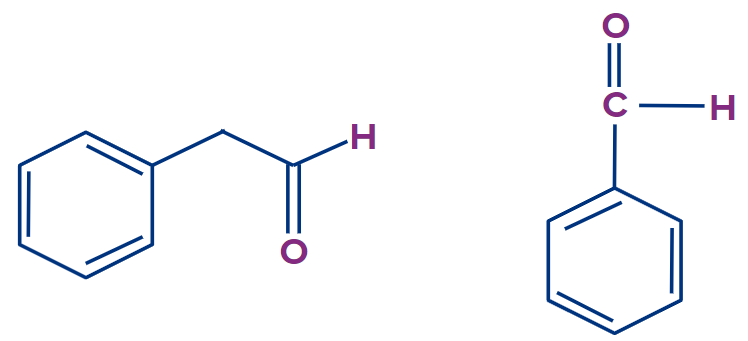
Phenylacetaldeyde will show tautomerism. Here X= -CH2 , y=carbon atom, Z= oxygen atom. whereas benzaldehyde won’t show tautomerism as there is no hydrogen atom with X.
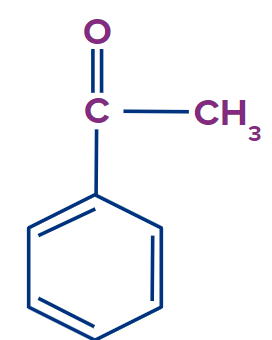
What do you think about acetophenone. I guess till now you must have understood the concept of deciding whether a compound will show tautomerism or not. Yes, this compound which is acetophenone will show tautomerism as there is a hydrogen atom in X
Here X= methyl group, y= carbon atom and Z= oxygen atom
Let’s discuss the condition of tautomerism in terms of presence of alpha hydrogen atom.
Alpha hydrogen atom
The first carbon atom that binds to a functional group, such as a carbonyl, is referred to as the alpha carbon in organic compounds.
The carbon that is directly connected to the functional group is called carbon. The hydrogen bonded to the carbon is referred to as hydrogen.
CH3-CH2-OH
In this compound, carbon atom of CH2 is the carbon and the two hydrogen atoms attached to it referred as hydrogen.
Ketones which have hydrogen atoms can show tautomerism.
Let’s discuss some of the exceptional cases of tautomerism
Example:
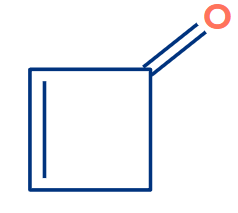
If you look closely, you will notice that there are two X. as discussed earlier if there are two X then try to consider that X which has sp3 hybridisation. Even if this compound satisfies the condition of X, Y and Z
still, it can’t show tautomerism.
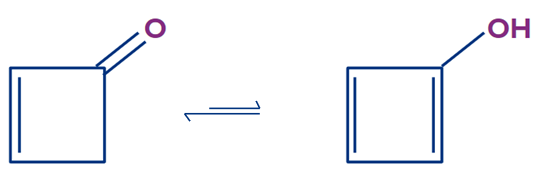
The enol form obtained from the above compound is the most unstable compound and does not exist.
This enol is anti-aromatic in nature. Let’s understand what is aromatic, antiaromatic and non-aromatic.
Not all ketone show tautomerism only those ketone who has alpha hydrogen will show tautomerism.
Aromaticity
All the cyclic compounds in organic chemistry either exist as aromatic, anti aromatic and in non-aromatic form. There is a special condition for each category.
Condition for a compound to be aromatic
We can only consider a compound to be aromatic if it satisfies the following conditions
- It should be a cyclic a compound
- It should be planar (lies in one plane)
- Pie electrons in these compounds are delocalised by conjugation
- It should obey huckel’s rule that is total number of pie electrons in these compounds should be in the order of (4n+2)
Or (4n+2)
Where, n= 1,2,3,4......n
Condition for a compound to be antiaromatic
We can only consider a compound to be anti-aromatic if it satisfies the following conditions
- It should be a cyclic a compound
- It should be planar (lies in one plane)
- Pie electrons in these compounds are delocalised
- pie electrons in these compounds should be in the order of (4n)
Or (4n)
Where, n= 1,2,3,4......n
Condition for a compound to be non-aromatic
We can only consider a compound to be non-aromatic if it satisfies the following conditions
- It should be a cyclic a compound
- Pie electrons in these compounds are not delocalised
- pie electrons in these compounds should be in the order of (4n+2)
Or (4n+2)
Where, n= 1,2,3,4......n
Note that non-aromatic compounds can show tautomerism but not that much as compared to aromatic compound whereas anti-aromatic compound can’t show tautomerism.
In the above compound you can see, it is cyclic, planar, conjugated and having 4 pie electrons. Which satisfies all the condition of an anti aromatic compound. Hence, it is anti-aromatic and most unstable compound.
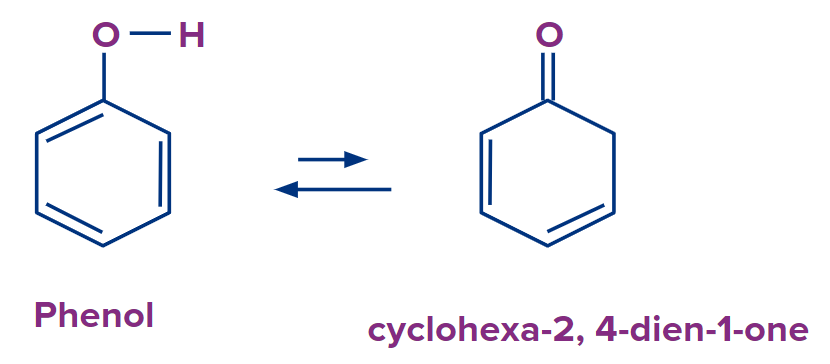
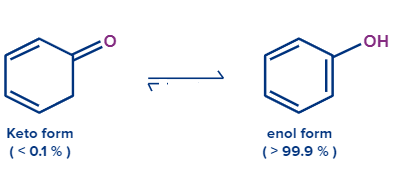
Let’s try to guess which compound will be more stable. Did you try? Well, then phenol will be formed 99.8% whereas cyclohexa-2,4dien-1-one will form only 0.2%. cyclohexa-2,4 dien-1-one is a non-aromatic organic compound as it does not have conjugated pie electrons.
It is interesting to note that antiaromatic compound will never show tautomerism, whereas non-aromatic compound will show tautomerism but at a very low extent.
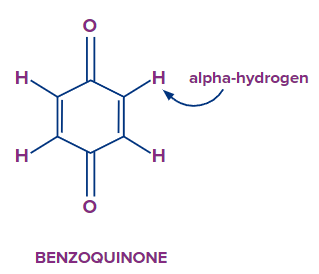
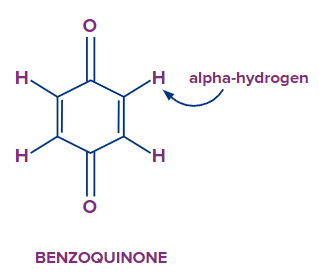
Let’s look at a special case of quinone or benzoquinone. This compound is aromatic in nature still it does not undergo tautomerism. You can see from the above figure that there are two carbons which have alpha hydrogen atoms. If 1,3 Hydrogen migration occurs then the compound formed will have two adjacent double bonds, which is very unlikely situation for a cyclic compound. As you know, if two adjacent double bonds are there then the respective carbon atom will have sp hybridisation. The bond angle in sp hybridisation is 1800 but you can see clearly the bond angle in the above compound is not even close to that. Due to this strain, compound won’t exist.
Factors affecting Percentage of enol content
In most of the cases percentage of keto form is more than enol form. But there are certain conditions under which the percentage enol content is more than that of keto form.
- Presence of hydrogen bonding
Due to the formation of hydrogen bonding enol form becomes more stable.
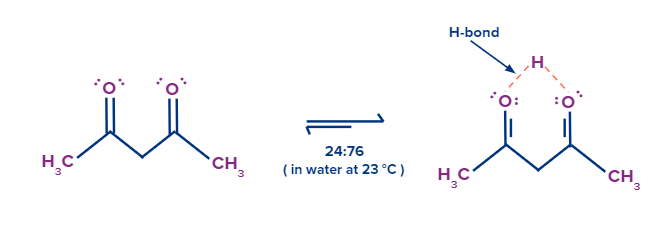
Here the enol form that is 4-hydroxy-3-penten-2-one is more stable due to the presence of hydrogen bonding. Hence, enol percentage is more than the keto form that is pent 2,4-dione
- Conjugation
Conjugation have a good contribution in the stability of an organic compound. If an electron withdrawing group is attached to the compound then the overall conjugation increases and so the stability of compound. If an electron donating group is attached to the compound then it will decrease the overall conjugation of the compound and so the stability.
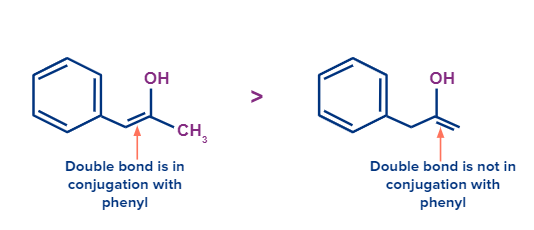
- Aromaticity
Aromatic stabilization favours the formation of enol content.
- Solvent
Solvent generally reverse the percentage of keto and enol form. Due to the formation of hydrogen bonding enol form becomes more stable in polar protic solvents.
Practice Problems
Q1. What will be the tautomer of acetone?
- Propanol
- Propanal
- Butenol
- isopropenol
Answer: (D)
Solution: Isomerism can be defined as a phenomenon where the same molecular formula exists in two or more interconvertible structures in a dynamic equilibrium. The structures in the dynamic equilibrium are called tautomers. Acetone undergoes 1,3 hydrogen shifts to give isopropenol or prop-2-enol.
Q2. Find out the reason why the below compound won’t show tautomerism?
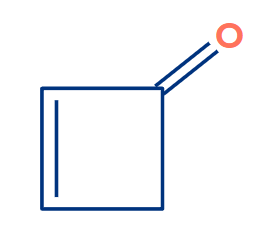
- Due to absence of alpha hydrogen atom.
- Due to anti-aromatic nature of enol form.
- Due to presence of carbonyl group.
- 4 membered cyclic compound doesn’t show tautomerism.
Answer: (B)
Solution: The enol form obtained from the above compound is the most unstable compound and does not exist. This enol is anti-aromatic in nature.
In the above compound you can see, it is cyclic, planar, conjugated and having 4 pie electrons. Which satisfies all the condition of an anti aromatic compound. Hence, it is anti-aromatic and most unstable compound.
Q3. Why does pivaldehyde does not show tautomerism?
- Due to absence of alpha hydrogen atom.
- Due to anti-aromatic nature of enol form.
- Due to presence of carbonyl group.
- 4 membered cyclic compound doesn’t show tautomerism.
Answer: (A)
Solution: The compound must have a structure X-Y=Z to show tautomerism
X= any atom which contains hydrogen but remember one thing it should not be hydrogen only.
Y= any atom which can form double bond
And Z= It should be either carbon, oxygen, nitrogen or sulphur
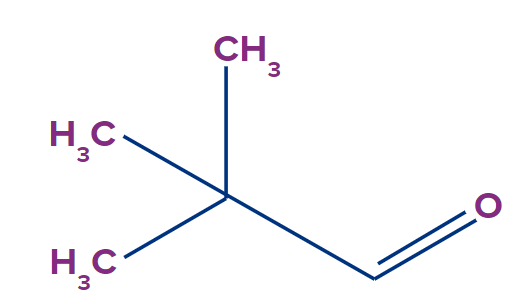
In case of Pivaldehyde, X does not have hydrogen atom. Hence, this compound won’t show tautomerism.
Q4. Which of the following compound/compounds will show tautomerism?
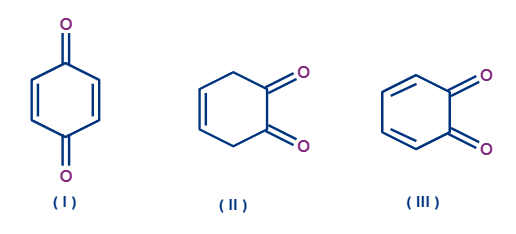
- iii
- ii
- i & iii
- All of the above
Answer: (B)
Solution: Structure (i) is aromatic in nature still it does not undergo tautomerism. You can see from the figure that there are two carbons which have alpha hydrogen atoms. If 1,3 Hydrogen migration occurs then the compound formed will have two adjacent double bonds, which is very unlikely situation for a cyclic compound. As you know, if two adjacent double bonds are there then the respective carbon atom will have sp hybridisation. The bond angle in sp hybridisation is 1800 but you can see clearly the bond angle in the above compound is not even close to that. Due to this strain, compound won’t exist.
Structure (iii) is anti-aromatic in nature which is highly unstable hence it won’t show tautomerism.
Structure (ii) is aromatic and has a stable conjugation of pie electrons. Hence, it will show tautomerism
Frequently asked question?
Q1. Can an aldehyde show tautomerism?
Answer: Yes, any compound which has alpha hydrogen atom and have the following structure can show tautomerism.
X-Y=Z
X= any atom which contains hydrogen but remember one thing it should not be hydrogen only.
Y= any atom which can form double bond
And Z= It should be either carbon, oxygen, nitrogen or sulphur
Remember it is not only confined to keto-enol tautomerism.
Q2. Is there any formula to determine percentage enol content?
Answer: No, there’s no such formula to determine the percentage of enol content. It is purely based on the observation and data of laboratory. We can only compare between two compounds to know which one will be formed in a major quantity.
Q3. What will be the priority if a compound is showing both functional isomerism and tautomerism?
Answer: In this situation, the priority must be given to tautomerism. The priority order of isomerism is
RTFMCP .
R= Ring chain isomerism
T= Tautomerism
F= Functional isomerism
M= Metamerism
C = chain isomerism
P = position isomerism
Q4. What distinguishes tautomerism from metamerism?
Answer: The major distinction between tautomerism and metamerism is that metamerism refers to structural isomerism in which alkyl groups that are connected to the same functional group are different, and the latter refers to dynamic equilibrium between two compounds with the same molecular formula. Tautomerization refers to the isomerization of tautomers, whereas mesmerization refers to the isomerization of metamers.


