-
Call Now
1800-102-2727
Sulfonation - Electrophilic Aromatic Substitution, Sulfonation of Benzene, its Mechanism, Practice Problems & FAQs
We are all aware that today we typically need good detergents in order to remove a difficult stain.
Right?
How do they perform while washing?
Applying detergent and then rinsing with fresh water are the two steps needed to remove a stubborn stain. The rinse cycle aids in clearing away loose dirt and other mud, but it may also aid in removing particles that have become lodged in the fabric's tiny pores.

Do you want to know what kinds of chemicals are used?
Surfactants, which are molecules that facilitate the mixing of water and other substances like oil or grease, are present in detergents and other cleaning supplies. Benzenesulfonic acid salts like Monoethanolamine benzenesulfonate and Sodium benzenesulfonate are surfactants found in laundry detergent.
Table of Contents
- Electrophilic Aromatic substitution
- Sulfonation of Benzene
- Mechanism
- Practice Problems
- Frequently Asked Questions
Electrophilic Aromatic substitution:
In organic reactions known as electrophilic aromatic substitutions, an atom bonded to an aromatic ring is replaced by an electrophile. Typically, in these reactions, an electrophile takes the place of a hydrogen atom from a benzene ring.
An electrophilic aromatic substitution process maintains the aromaticity of the aromatic system. The stability of the aromatic ring is retained when benzenesulfonic acid is produced from the reaction of benzene and sulfuric acid. The following example demonstrates this response.
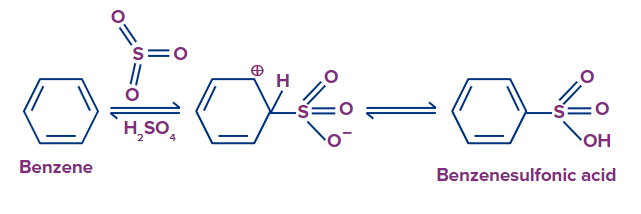
Sulfonation of Benzene:
It is an electrophilic aromatic substitution reaction in which SO3 act as an electrophile. In this reactions, benzene is heated in the presence of fuming sulfuric acid (H2SO4+SO3) to create benzene sulfonic acids. The process of the reaction can be reversed.
Mechanism:
Step - 1: The first step of this reaction involves the generation of electrophiles from fuming sulphuric acid. By losing a water molecule from sulphuric acid, sulfur trioxide is formed
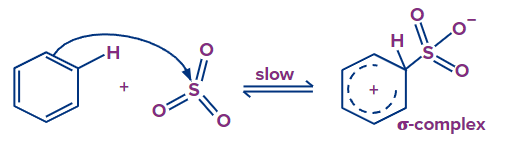
Step - 2: In the second step, oxygen in sulfuric acid attracts an electron because it has a higher electronegativity, creating an electrophile. The electron of the benzene ring is abstracted by electrophile SO3 and there is a formation of a sigma complex -complex. This step is the slow step and the rate-determining step of this reaction.
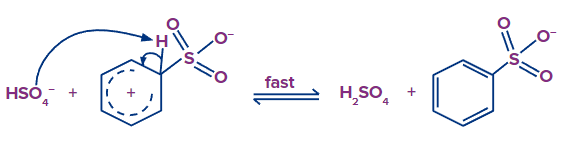
Step - 3: In the third step, the hydrogen attached to carbon having a sulfonic group is eliminated by HSO4- formed in the first step and there is a formation of benzenesulfonate.
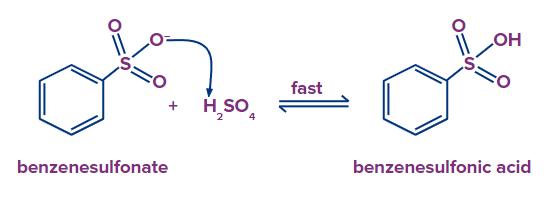
Step - 4: In the fourth step, hydrogen from the H2SO4 is abstracted by benzenesulfonate and there is a formation of Benzenesulfonic acid in the final.
Practice Problems:
Q1. What is the nature of the reaction when fuming sulphuric acid reacts with benzene to give benzenesulfonic acid?
- Nucleophilic Aromatic substitution reaction
- Electrophilic Aromatic substitution reaction
- Electrophilic Aromatic stabilization reaction
- Electrophilic Aliphatic substitution reaction
Answer: (B)
Solution: Electrophilic Aromatic substitution reactions is the nature of the reaction when fuming sulphuric acid reacts with benzene to give benzenesulfonic acid.
Hence, option (B) is the correct answer.
Q2. Which of the following steps is the rate-determining step in the electrophilic aromatic substitution reaction of benzene and fuming sulphuric acid?
- The step of reaction involves the generation of electrophiles from fuming sulphuric acid.
- The step involves the electron of the benzene ring being abstracted by electrophile SO3 and there is a formation of a sigma complex -complex.
- The step in which there is a formation of benzenesulfonate.
- The step in which hydrogen from the H2SO4 is abstracted by benzenesulfonate and formations of Benzenesulfonic acid.
Answer: (B)
Solution: This reaction begins with the production of electrophiles from fuming sulfuric acid. Sulfur trioxide is created when sulfuric acid loses a water molecule.
When an electrophile abstracts an electron from a benzene ring, a sigma complex is formed, and this is the second step in the process of creating an electrophile. This step is the slowest step and rate-determining step of this reaction.
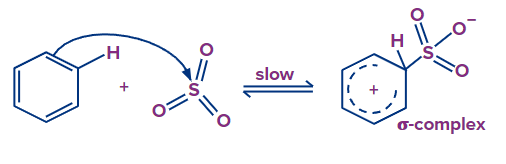
The formation of benzene sulfonate occurs in the third step, which also involves the elimination of the hydrogen attached to a carbon that has a sulfonic group that was created in the first step.
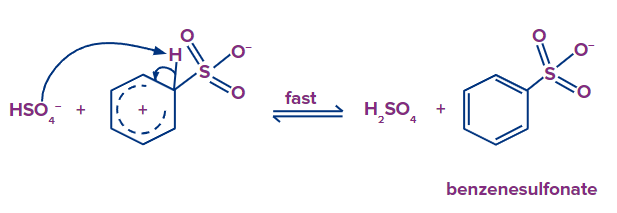
Benzenesulfonate absorbs hydrogen from H2SO4 in the fourth step, resulting in the formation of benzenesulfonic acid in the final step.

Hence, The step involves the electron of the benzene ring being abstracted by electrophile SO3 and the formation of a sigma complex -complex is the slowest and rate-determining step.
Hence, the correct answer is option (B).
Q3. What should be the expected product in the major in reaction when phenol is treated with fuming sulphuric acid?
- 4-hydroxy benzene sulfonic acid
- 2-hydroxy benzene sulfonic acid
- Both (A) and (B)
- Reaction do not take place
Answer: (A)
Solution: It is an electrophilic aromatic substitution reaction in which SO3 act as an electrophile. When phenol is heated in the presence of fuming sulphuric acid (H2SO4+SO3), there is a formation of both 4-hydroxy benzene sulfonic acid (para-hydroxy benzene sulfonic acid) and 2-hydroxy benzene sulfonic acid (ortho-hydroxy benzene sulfonic acid) as the hydroxy group is ortho-para directing group. But 4-hydroxy benzene sulfonic acid is formed as a major product because the SO3H group is bulky and creates less steric hindrance at the para position than at the ortho position.
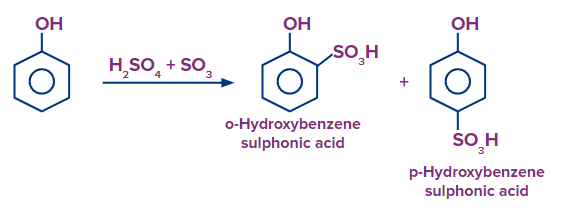
Hence, option (A) is the correct answer.
Q4. What should be the expected product formed in major when chlorobenzene is treated with fuming sulphuric acid and benzenesulfonic acid is treated with Cl2/FeCl3 respectively ?
- 4-chlorobenzenesulfonic acid and 4-chlorobenzenesulfonic acid
- 2-chlorobenzenesulfonic acid and 2-chlorobenzenesulfonic acid
- 2-chlorobenzenesulfonic acid and 3-chlorobenzenesulfonic acid
- 4-chlorobenzenesulfonic acid and 3-chlorobenzenesulfonic acid
Answer: (D)
Solution: It is an electrophilic aromatic substitution reaction in which SO3 act as an electrophile. When chlorobenzene is heated in the presence of fuming sulphuric acid (H2SO4+SO3), there is a formation of both 2-chloro benzene sulfonic acid (p-chloro benzene sulfonic acid) and 4-chloro benzene sulfonic acid (o-chloro benzene sulfonic acid) as the chloro group is ortho-para directing group. But 4-chloro benzene sulfonic acid is formed as a major product because the SO3H group is bulky and creates less steric hindrance at the para position than at the ortho position.
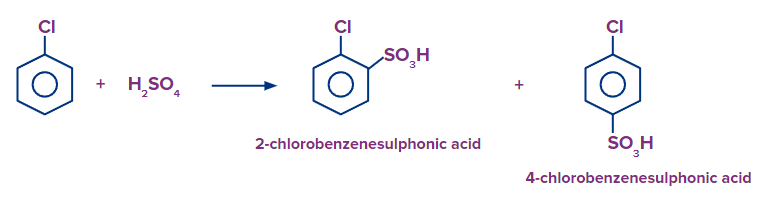
When benzene sulfonic acid is heated in the presence of Cl2/FeCl3, there is a formation of 3-chloro benzene sulfonic acid (m-chloro benzene sulfonic acid) as the sulfonic group is a meta directing group.
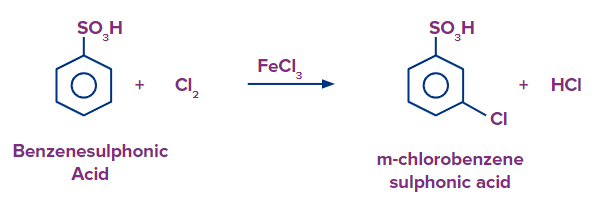
Hence, the products formed are 4-chloro benzene sulfonic acid and 3-chloro benzene sulfonic acid, respectively and the correct answer is option (D).
Frequently Asked Question-FAQs:
1. What is a substitution reaction?
Answer: A substitution reaction is when an atom or group of atoms in an organic molecule are directly replaced by another atom or group of atoms without causing any changes to the remaining components of the molecule.
2. What is the definition of the electrophilic and nucleophilic substitution reaction?
Solution: The name "electrophilic substitution reactions" refers to the fact that they involve substitution and start with an electrophile attack.
A nucleophilic aromatic substitution is a type of substitution process used in organic chemistry that involves the nucleophile dislodging a good leaving group, such as a halide, from an aromatic ring.
3. What does the term "electrophilic addition reaction" mean?
Answer: Electrophilic addition reactions are those in which two reactive molecules combine to form a single molecule of the product. These reactions are started by the electrophilic component of the reagent.
4. What exactly are electrophiles and nucleophiles?
Answer: An attacking reagent with love or attraction for the nucleus or positive center is known as a nucleophile. As a result, it is either negatively charged or neutral, but it has at least one pair of easily donatable electrons. An assault substance that loves or attracts electrons is known as an electrophile. Therefore, it is either positively charged or neutral but lacking in electrons.


