-
Call Now
1800-102-2727
Structural Isomerism – Isomerism, its Classification, Structural Isomerism, Practice Problems and FAQ
Do you know the process used to make elegant cups, saucers, and lamps?
Clay is the substance used to create these incredible works of art. Pottery is the name for the craft of creating objects out of clay. Clay may be moulded into a variety of shapes.
However, what role does pottery play on this concept page?
There are similarities and significance here. In chemistry, there are molecules with the same molecular formula but diverse structures, just like how clay may be formed into several shapes. Structural isomers are molecules with the same chemical formula but distinct structures.
We will learn more about isomerism and its classification on this concept page, with a focus on structural isomerism.
TABLE OF CONTENTS
- Isomerism
- Isomerism – Classification
- Structural Isomerism – Introduction
- Structural Isomerism - Types
- Practice Problems
- Frequently Asked Questions – FAQ
Isomerism
The phenomenon of the existence of two or more compounds possessing the same molecular formula but
Structural formula, physical and chemical properties is known as isomerism. Such compounds are known as isomers.
The word ‘Isomer’ is derived from two Greek words “isos” and “meros”, which mean “equal” and “parts” respectively.
Example: Pentane, 2-methylbutane and 2,2-dimethylpropane are isomers of each other. They have the same molecular formula (C5H12) but have different structural formulas, and physical and chemical properties.
Isomerism – Classification
Isomerism can be broadly classified into two types namely structural isomerism and stereoisomerism.
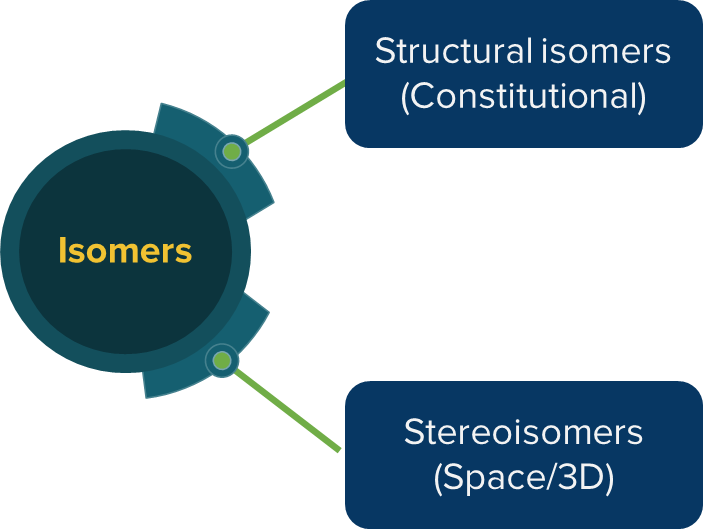
Structural Isomerism: Compounds having the same molecular formula but different structural formulas i.e. they differ in the bonding sequence of atoms, are called structural isomers and the phenomenon is called structural isomerism. Structural isomers are also called constitutional isomers.
Example:

Stereoisomerism: Isomers that have the same bonding sequence of atoms and groups but differ from each other only in the way in which their atoms are oriented in space are called stereoisomers and the phenomenon is called stereoisomerism.
Example:

In this concept page, we will focus mainly on structural isomerism.
Structural Isomerism – Introduction
Structural isomerism is the phenomenon where chemical compounds have the same molecular formula but different chemical structures. Structural isomers differ in the functional group or the atom linked to the central atom or both. Structural isomerism is also called constitutional isomerism. In the structural isomers, the molecular formula remains the same though the functional group may or may not be the same.
Structural Isomerism - Types
Structural isomers can be classified into
Chain Isomerism: The compounds that have the same molecular formula but different carbon skeletons or parent chains are called chain isomers and the phenomenon is called chain isomerism. In simple terms, the compounds containing the same molecular formula, but a different number of carbon atoms in their parent chain are called chain isomers.
Examples:
Positional Isomerism: Compounds having the same size of the main chain and the side chain along with the same nature of the functional groups, but having a difference in the position of multiple bond/ functional group/substituent are called positional isomers and the phenomenon is called positional isomerism.
Examples:
Functional Isomerism: Compounds having the same molecular formula but different functional groups are called functional isomers and the phenomenon is called functional isomerism.
Examples:
Ring-Chain Isomerism: The isomerism in which one isomer has a ring while the other has an aliphatic chain is called ring-chain isomerism and the isomers are called ring-chain isomers. Ring-chain isomers are also functional isomers.
Examples:


Metamerism: Compounds having the same nature of functional groups but different natures of alkyl groups along the polyvalent functional group are called metamers and the phenomenon is called metamerism. The polyvalent functional groups that can show metamerism are given below.
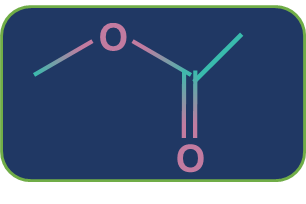
Ester
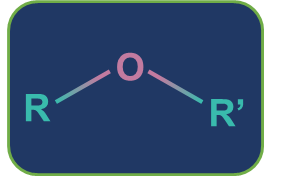
Ether
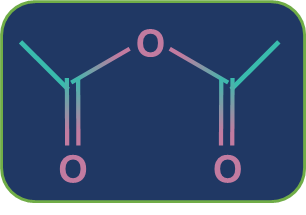
Acid anhydride
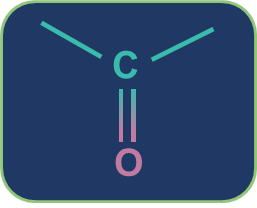
Ketone
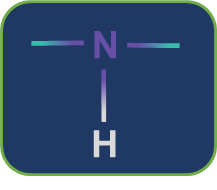
Secondary amine
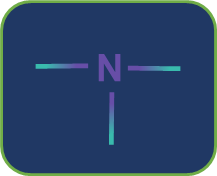
Tertiary amine
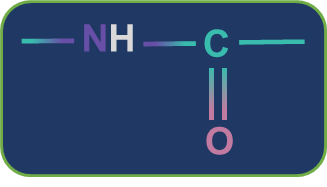
Secondary amide
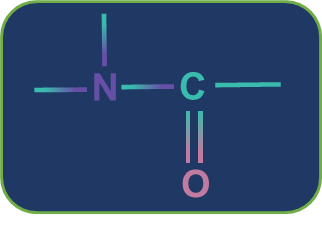
Tertiary amide

Thio ether
Examples:
Tautomerism: The phenomenon by which a single compound exists in two or more readily interconvertible structures that differ in the relative positions of at least one atomic nucleus,
generally hydrogen, is called tautomerism and the two intervonvertible structures are called tautomers. Tautomerism occurs in only a few chemical compounds and is caused by intramolecular proton transfer.
Tautomers exhibit in dynamic equilibrium with each other.
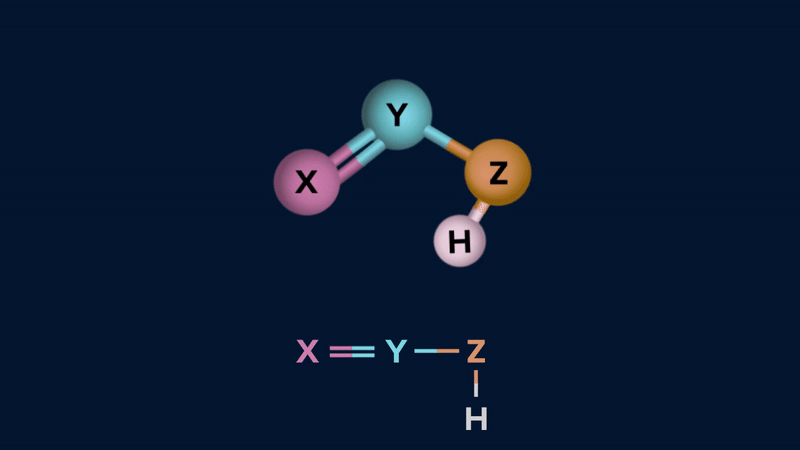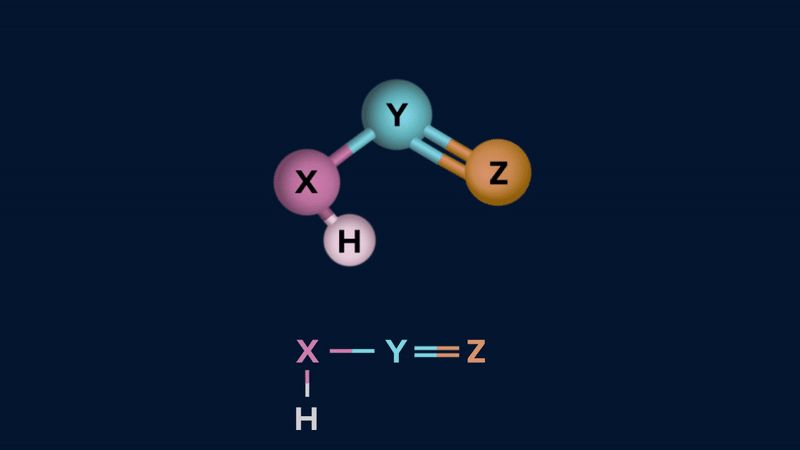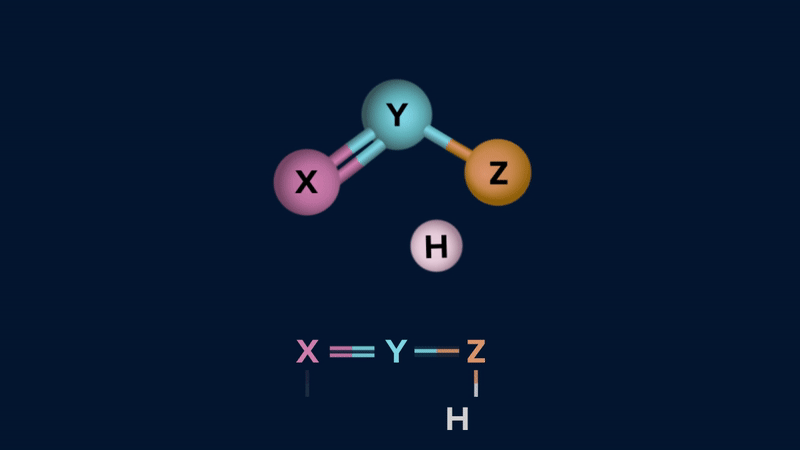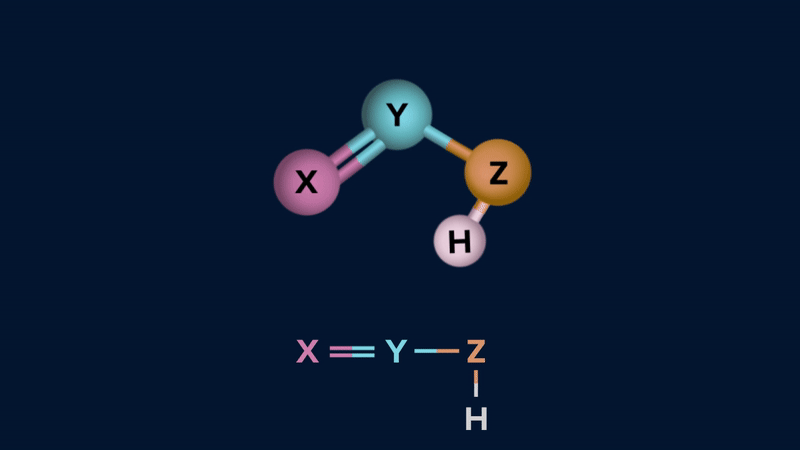
Tautomerism can be of two types.
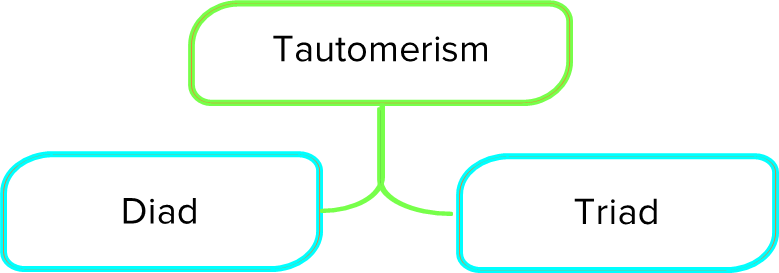
Diad: The movement of hydrogen between the 1 and 2 positions is called diad tautomerism.
Triad: The movement of hydrogen between the 1 and 3 positions is called triad tautomerism.
One of the most famous examples of triad tautomerism is the keto-enol tautomerism.
Conditions for tautomerism:
- Tautomerism takes place in any of the given functional groups.
- There should be atleast one hydrogen atom attached to the sp3 hybridised -carbon atom.
- Tautomerism is possible if an ⍺, ꞵ unsaturated carbonyl compound contains a hydrogen atom at its sp3 hybridised -carbon atom.
Keto-Enol Tautomerism
Keto-enol tautomerism is a form of tautomerism between a carbonyl compound containing ⍺-H and its enol form.
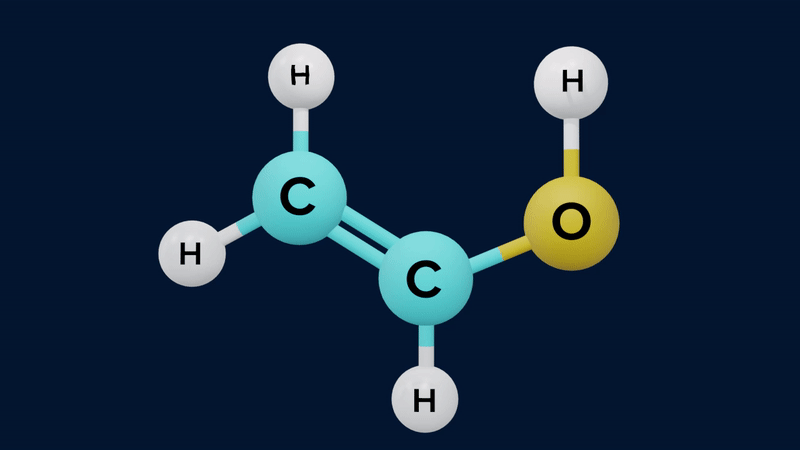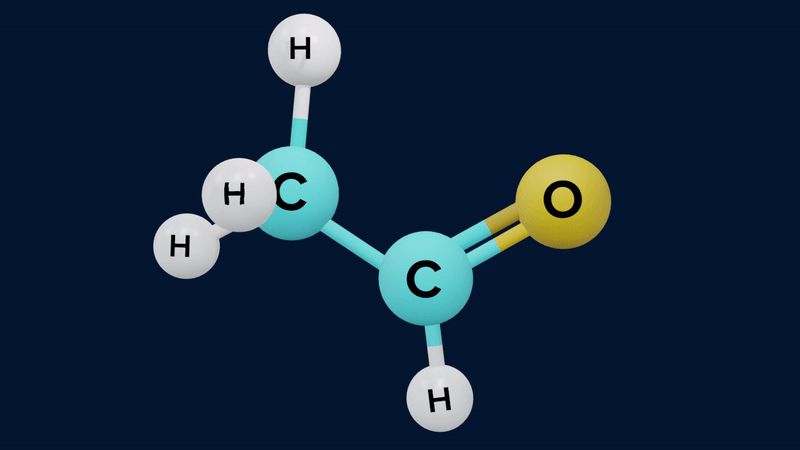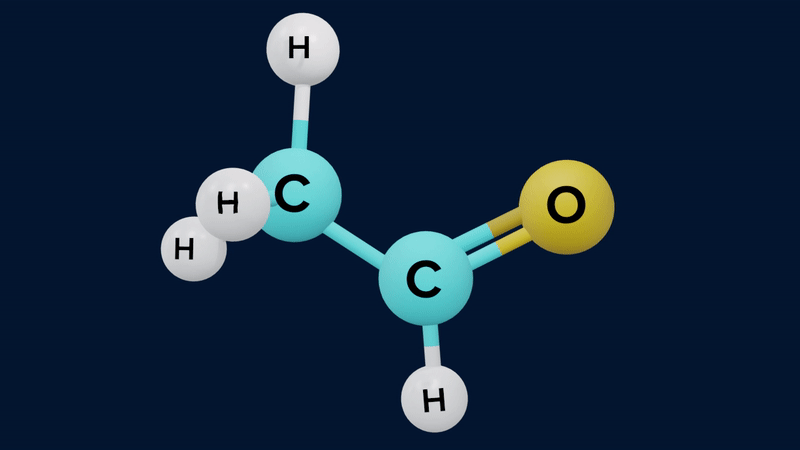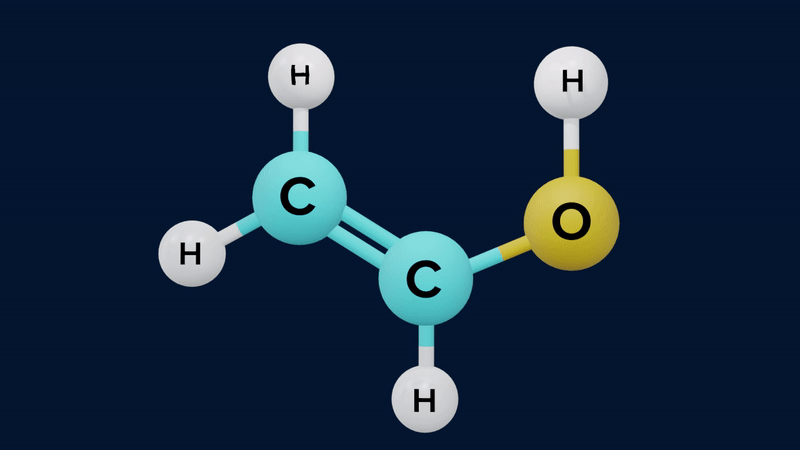
The keto form of mono carbonyl compound is more stable than its enol form. This is because the keto form differs from the enol form in possessing a C-H, a C-C and a C=O bond, where the enol has a C=C, a C-O, and an O-H bond. The approximate sum of the first three is and of the second three is . The keto form is therefore thermodynamically more stable by .
Usually, the keto form of acyclic 1,2-Dicarbonyl compound is more stable than its enol form.
The enol form of cyclic 1,2 Dicarbonyl compound is more stable than its keto form.
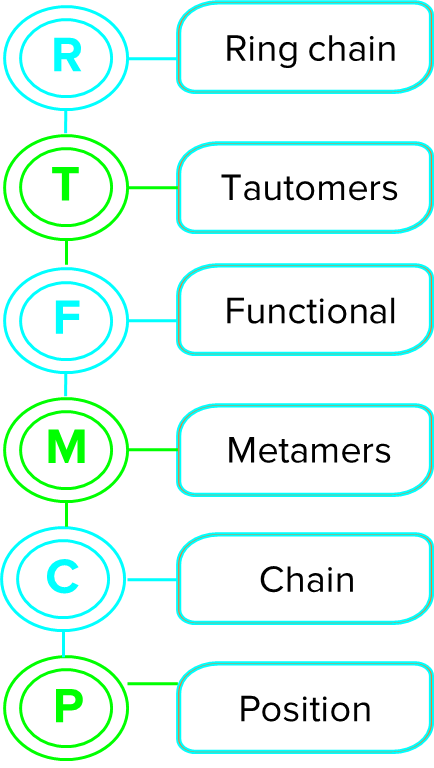
If there are numerous varieties of isomerism, how do you choose one?
The order of preference of isomerism is given below.
Recommended Videos
Isomerism: General Organic Chemistry (GOC) | Class 11 Chemistry Chapter 12 | JEE Main 2022 Exam Prep
Isomerism in Organic Compounds Class 11 Chemistry (Concepts & Imp Questions) | JEE Main 2022 /2023
Practice Problems
Q1. Which of the following pairs of molecules may represent isomers?
A. Pentane and butane
B. Ethane and ethene
C. Butane and 2-methyl propane
D. Cyclopropane and butane
Answer: C
Solution: Isomerism is the phenomenon in which two or more compounds have the same chemical formula but differ in one or more physical or chemical properties. Chemical compounds have identical formulae but different physical or chemical properties are named isomers. Butane and 2-methyl propane have the same molecular formula of C4H10 and therefore it may represent an isomeric pair but in other options, the molecular formula of compounds is different and cannot be considered as an isomeric pair.
So, option C is the correct answer.
Q2. Find the relation between the given compounds.
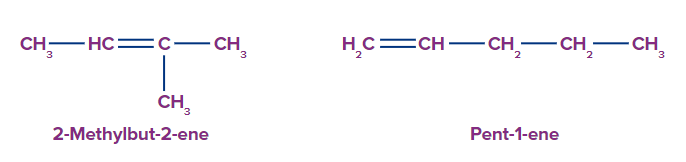
A. Functional isomerism
B. Positional Isomerism
C. Both (A) and (B)
D. Chain Isomerism
Answer: D
Solution:
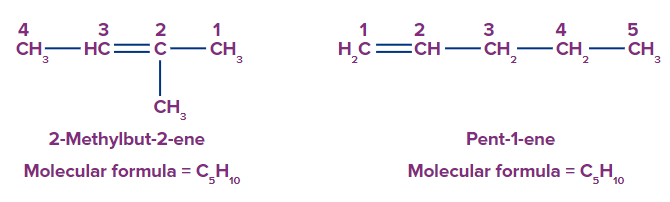
- 2-Methylbut-2-ene and pent-1-ene have the same molecular formula, C5H10.
- 2-Methylbut-2-ene has four carbon atoms in the parent chain.
- Pent-1-ene has five carbon atoms in the parent chain.
Since the parent chain is different for both of these compounds, they are chain isomers.
So, option D is the correct answer.
Q3. Consider the given set of compounds: pentane, butane, 2-methyl butane, propane. The number of pairs of isomers possible will be_____.
A. 1
B. 2
C. 4
D. 3
Answer: A
Solution: Chemical compounds have an identical formula but different physical or chemical properties are named isomers. Therefore, only pentane and 2-methyl butane can show isomerism because they have the same chemical formula (C5H12) and the number of possible pairs is equal to 1.
So, option A is the correct answer.
Q4. Which type of isomerism is exhibited by 1-butanol and butan-1,2-diol molecule?
A. Chain isomerism
B. Functional isomerism
C. Positional isomerism
D. Will not show isomerism
Answer: D
Solution: As we know for a compound to exhibit isomerism it should have the same molecular formula. But in the case of 1-butanol (C4OH9) and butan-1,2-diol (C4O2H10) molecules, the molecular formulae are different and therefore it will not exhibit the isomerism.
So, option D is the correct answer.
Frequently Asked Questions – FAQ
Q1. What is the difference between constitutional and configurational isomerism?
Answer: Constitutional or structural isomerism is the phenomenon where chemical compounds have the same molecular formula but different chemical structures. Structural isomers differ in the way functional groups and other atoms are linked to the central atom. Whereas, configurational isomerism is a type of isomerism in which isomers differ in configuration i.e. the spatial arrangement of atoms is different.
Q2. In which functional groups functional isomerism is possible?
Answer: Functional group isomerism is possible in those compounds which have the functional groups such as alcohols and ethers, aldehydes and ketones, cyanides and isocyanides, acid and ester, etc.
Q3. Does alcohol show functional isomerism?
Answer: Functional isomers include alcohols and ethers. In ether, both the hydrogen atoms are swapped by an alkyl group, while one hydrogen atom is swapped by an alkyl group in alcohol. Alcohol therefore exhibits functional isomerism.
Q4. Why is rotation along double-bonded atoms difficult as compared with the single-bonded atoms?
Answer: Rotation around a single bond is easy, but it is difficult to rotate around a double bond. This is because, as the electrons overlap both above and below the plane of the atoms, the rotation of pi bond is restricted.


