-
Call Now
1800-102-2727
Steam Distillation: Process, Principle, Process, Extraction of Essential Oils, Applications, Advantages, Practice Problems and FAQs
Sandalwood fragrance is very pleasant and most preferred in spiritual activities. The sandalwood oil finds use in the making of incense sticks, cosmetics, soaps, Ayurvedic aromatherapy etc. But do you know how this oil is extracted from the barks and other parts of the sandal tree?
The simplest way of getting this sandalwood oil is by steam distillation. Steam distillation is useful in obtaining essential oil from plants. But what is this steam distillation and how is it different from other distillations? You will learn them in this description.
At extremely high temperatures, many organic compounds can decompose. A distillation process would be destroying the compound and will ot be useful to separate the components in pure form. In steam distillation,distillation is done at lower temperatures. If the substance is very sensitive to heat, steam distillation can be used. The vapour is condensed after the distillation process
Table of content:
- Steam Distillation and Conditions
- Principle of Steam Distillation
- Steam Distillation Process
- Distillation of Essential Oils
- Applications
- Advantages
- Practice Problems
- Frequently Asked Questions
Steam Distillation
Every substance has its own boiling point. Simple distillation is a process of separating pure liquid from a mixture of liquids differing at least by 25°K in their boiling point. If the difference is less than that fractional distillation having separate columns has to be employed. But there are liquids which are high boiling and that to get decomposed at their boiling points. Such substances cannot be heated to their boiling points for purification. Such liquids can be separated using steam distillation from their contaminants at temperatures much lower than their boiling point without any decomposition.
Distillation of a mixture with the help of steam to get pure material is referred to as steam distillation.
The liquid to be distilled can be miscible or better immiscible with water but should have sufficient vapour or vapour pressure at steam temperature for steam distillation separation.
Steam distillation is useful for -
- High boiling point liquids which are immiscible with water
- Liquids that are not stable at or near their boiling point
- Essential oils and volatile aroma compounds..
Principle of Steam Distillation:
Every substance has some of its atoms or molecules as vapour above it, creating a vapour pressure. Amount of vapour or their vapour pressure differs from substance to substance.being high for low boiling and extremely less for solids. The substance starts boiling when the vapour pressure equals the atmospheric pressure. Formation of vapour increases with increasing the temperature by heating.
A high boiling liquid requires high temperature to reach the atmospheric pressure.
A steam already has its vapour pressure equal to the atmospheric pressure. Such steam when flowing carries with it all vapours on its way. Removal of the vapour of the high boiling liquid will enhance the vapourization of the liquid and ultimately separation of the high boiling liquid at steam temperature which is much lower than the liquid.
Therefore, in the presence of water, liquids with high boiling points begin to vaporise at low temperature, forming a mixture with water. Water does not dissolve many organic substances and are immiscible with them. Rather, it forms separate layers on cooling. On passing steam through the high boiling organic mixture, steam carries with it the vapours of the organic substance, which can be cooled and separated from the immiscible layer using a separating funnel. As a result, they can be separated at an absolute temperature lower than their decomposition temperature.
Water can be used for distilling of high boiling liquid in two ways
- Boiling with water and
- Passing steam through the high boiling liquid or solid.
The liquid is mixed with water and boiled together. This distillation is based on the principle that when two immiscible mixtures are heated together, each liquid works magically in the presence of other substances, significantly increasing the vapour pressure in the system as the sum of the vapour pressures of all the components of the mixture. So, boiling and distillation become possible at lower temperatures than that of the individual components. The immiscible liquids can be separated by a separating funnel.
In steam distillation, high temperature steam is passed through the liquid and the vapour is collected in a separate chamber or flask.Steam has enough vapour that can flow out of the flask or chamber. Steam, while flowing out, can carry along with it any volatile material with even low vapour over the liquid, at much lower temperatures. The advantage with steam distillation over boiling with water, is that higher temperature than the boiling point can be used for quicker distillation and since the steam already has high vapour pressure even liquids with low vapour can be separated in good amounts.
Steam Distillation Process:
Water is heated to steam in a separate chamber. The steam is passed through the solid or liquid mixture in an another heated vessel. The vaporised mixture of water vapour and organic compounds flowing through a cooling column and is condensed.
The cooled liquid mixture is received in a separate vessel and separated with a separating funnel if the liquid is immiscible with water or distilled if miscible.
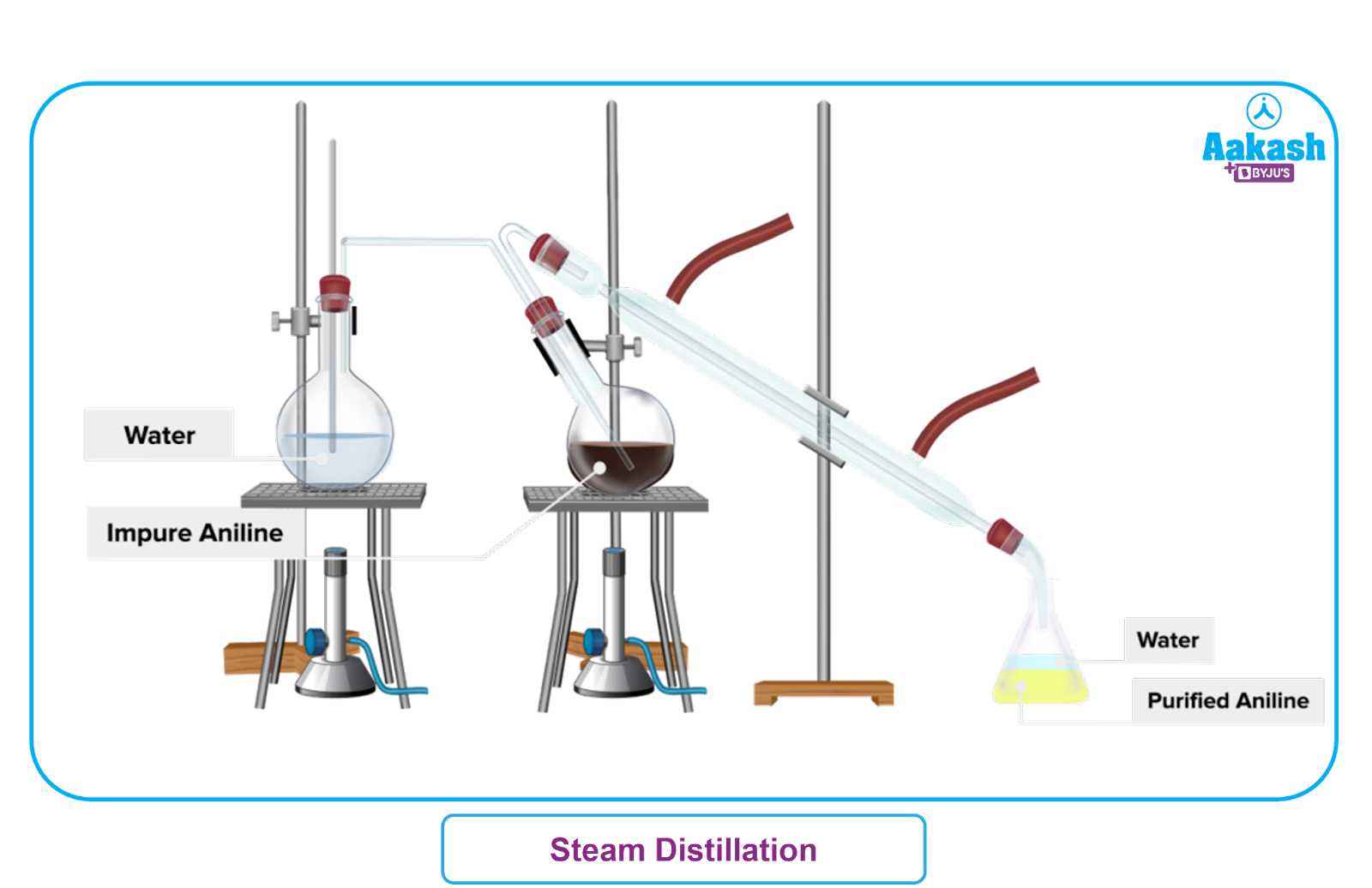
Distillation of Essential Oils:
The extraction of essential oils in the form of aromatic compounds from various plant species is simple and effective when done through steam distillation. The steam flows through the plant parts in the flask or chamber before being gently released into the appliance. This steam has the ability to release oil and replace volatile substances.
Applications of Steam Distillation:
- The process of steam distillation is frequently employed in the isolation of natural oils and fragrances. By using this technique, natural fragrance compounds from, lemon grass , oranges peels and eucalyptus leaves etc are isolated in large quantities.
- Used in decomposing and removing ammonia present in effluents of industries.
- Removal of toxic chemicals like benzene from the mixture.
- Steam distillation is used to separate alcohols ,esters produced from the petroleum products. .
Advantages of Steam Distillation:
- It does not necessarily require a large amount of fuel or energy.
- Very economical as it does not require maintenance-intensive equipment
- Create products without the use of organic solvents
- Easy to extract essential oils.
Practice Problems:
Q.1. A lot of organic substances do not dissolve in-
(A) Ether
(B) Water
(C) Alcohol
(D) Benzene
Answer: (B)
Solution: All organic compounds, including alkanes, alkenes, alkynes, and alkyl halides, are slightly soluble in water. Water is usually soluble in alcohols, carboxylic acids, amines, and esters. However, as the molecular weight of these compounds increases, so does their water solubility. As a result, the majority of organic compounds are insoluble in water.
Q.2. What is the primary factor that steam distillation depends on?
(A) Melting point
(B) Boiling point
(C) vapour pressure
(D) Temperature
Answer: (C)
Solution: When two immiscible mixtures are heated together, each liquid exerts its effect in the presence of other substances, significantly increasing the vapour pressure in the system. The two liquids begin to boil and the vapour pressure of these liquids exceeds atmospheric pressure. Therefore, steam distillation mainly depends on the vapour pressure.
Q.3. Which technique is applied to isolate plant organic compounds?
(A) Steam distillation
(B) sublimation
(C) Decantation
(D) Condensation
Answer: (A)
Solution: At a high temperature, some organic compounds decompose. Thus, steam distillation is a reliable method for removing organic compounds from plant species and extracting aromatic hydrocarbons.
Q.4. Substances that are decomposed by------- are separated by steam distillation
(A) Temperature
(B) pressure
(C) Volume
(D) Mass
Answer: (A)
Solution: At high temperatures, some of the organic compounds may decompose. For the separation of such temperate sensitive substances, steam distillation is used, as it allows the compounds to vaporise below their boiling point.
Frequently Asked Questions:
Q1.Why is para nitrophenol less volatile than ortho nitrophenol?
Answer: o-Nitrophenol forms an intramolecular H-bond, and p-Nitrophenol binds via an intermolecular H bond. During boiling, strong intermolecular hydrogen bonds raise the boiling point, more than the intramolecular hydrogen bonds on o-nitrophenol.. Therefore, o-Nitrophenol is more volatile than p-Nitrophenol.
Q2. What makes the three distillation processes different from one another?
Answer:
1) Distillation: This method is used to separate liquids with very different boiling points. Distillation is used when there are non-volatile impurities in a volatile liquid. A simple experimental setup with two simple flasks and a condenser is required. Used in the purification of seawater.
2) Fractional distillation: This method is used to distinguish between liquids with closer boiling points. Multiple simple and direct distillation processes are carried out with minimal losses during fractional distillation. A complex experimental set-up with a fractionating column apparatus is necessary. used to refine crude oil.
3) Steam Distillation: This method is used to clean up substances that are sensitive to temperature. This technique can be used to purify a liquid that breaks down when it is heated. Reduced pressure decreases the boiling point. Thus, decomposition is prevented. used to separate water from aniline.
Q3.Why does an organic liquid evaporate at a temperature below its boiling point in its steam distillation?
Answer: In steam distillation, the organic liquid starts to boil when the sum of vapour pressure due to the organic liquid (P1) and the vapour pressure due to water (P2) becomes equal to atmospheric pressure (P), that is, P=P1+P2
Since P1 < P2, the organic liquid will evaporate at a lower temperature than its boiling point.
Q4. What is steam distillation used for? Give an example of a mixture that is separated using steam distillation
Answer: Steam distillation is used for the separation of two immiscible liquids below their decomposition points. It is also used for extracting aromatic compounds from plant species. Steam distillation is used to separate a mixture of water and aniline.


