-
Call Now
1800-102-2727
Solvent Extraction: Introduction, Principle, Process, Selection of Solvents, Procedure, Applications, Practice Problems & Frequently Asked Questions
Have you seen a coffee-making machine? The machine powders the coffee beans and mixes them with hot water to give a black coffee liquid. Water acting as a solvent dissolves (extracts) and separates the water-soluble caffeine and the aroma from coffee beans.
Solvent extraction is a process of separation of a component from a mixture using a solvent.
Another example is the oils extracted from plant seeds using organic solvents like hexane, acetone, toluene etc. The leaching of metals like copper and gold is also an example of this process. The above examples are solid-liquid extraction processes.
There is also a solvent extraction process called the liquid-liquid extraction process. The liquid-liquid extraction process involves the separation of solute from a mixture using two immiscible solvents. Most of the refined edible oils are obtained by this liquid-liquid extraction process.
Table of Contents:
- Introduction of Solvent Extraction
- Principle of Solvent Extraction
- Selection of Solvents
- Procedure for Solvent Extraction
- Uses of Solvent Extraction
- Practice Problems
- Frequently Asked Questions(FAQs)
Introduction of Solvent Extraction:
You know that ionic compounds dissolve more in polar solvents while organic compounds dissolve more in nonpolar solvents. So, substances have different solubilities in different solvents.
Separating substances from a mixture based on their relative solubilities in two different immiscible solvents is known as liquid-liquid extraction (LLE), or partitioning extraction but generally as solvent extraction.
Immiscible liquids are those that, when shaken together, do not mix up but separate into distinguishable layers. Usually, water plus an organic solvent make up these liquids. In liquid-liquid extraction (LLE), the solutes get distributed between both solvents corresponding to their solubilities in them.
The distribution concept is most frequently applied both on a small scale in chromatographic identification or separation or in large-scale production. Organic solvents like benzene, chloroform, and ether, which are immiscible in water, are typically significantly more soluble in organic molecules than in water.
The water-organic solvent mixture splits into two layers when shaken. More organic compounds would flow into a non-aqueous layer because of their distribution ratio. The non-aqueous solution can be evaporated to leave behind the pure substance.
Principle of Solvent Extraction
Distribution Coefficient or Partition Coefficient:
It is based on the law of distribution. The law is often referred to as the Nernst distribution law. A solute X is said to distribute across two immiscible solvents A and B at a constant temperature if X is in the same molecular state in both solvents,then
Solutes frequently dissolve in part into both layers when a solution is placed in a separatory funnel and agitated with an immiscible solvent.
The components are said to be "partitioned" or "distributed themselves" between the two layers.
If chemical A is to be extracted from an aqueous solution and dissolved into an organic solvent, the equation for compound A's dissolution equilibrium can be represented as
When equilibrium is reached, the ratio of solute concentration in each layer remains unchanged for each system, and this can be expressed by the value KD.
Here, KD = distribution coefficient and it is also called partition coefficient .
The temperature of the system controls this equilibrium process (dissolution equilibrium).
Factors affecting solvent extraction:
The most popular techniques for commercial oil extraction are solvent extraction and mechanical pressing, but solvent extraction is more effective in terms of oil recovery. The kind of solvent used, meal particle size, extraction temperature, solvent to solid ratio, extraction time, amount of moisture content in the solid, and percentage of oil production can all have an impact on the yield of oil during solvent extraction. Oil from plant seeds can be extracted using a variety of solvents. The most widely used and documented oil extraction solvents include hexane, petroleum ether, diethyl ether, ethanol, n-heptane, isopropanol, acetone, chloroform, methanol, and 1-butanol. Various extraction solvents can have different oil extraction efficiency, environmental consequences, and renewability.
Additionally, it has been claimed that various solvents produce various natural chemicals from a given substance, leading to the possibility that the content of the extract will change depending on the solvent. As a result, choosing a solvent for oil extraction is one of the most crucial processes in the chemical extraction of oil. Due to its low boiling point, ease of recovery from the extract, and the fact that the majority of oils are soluble in hexane, hexane is generally the most used solvent for extracting oil from plant sources.
Selection of Solvents:
- The solute should have higher solubility or distribution coefficient in the organic solvent.
- The solvent shouldn't react with the solute
- The solvent's boiling point must be low enough (much below the solute's melting point) for easy evaporation after collection.
- It should have a good temperature coefficient.
Procedure for Solvent Extraction:
In laboratories, a separating funnel is used to collect the specified solute's aqueous solution. The desired organic solvent is added to it. The funnel is closed, and a vigorous shake is applied to its contents. It is then allowed to settle gently for a while. The solid or liquid solute will move from the aqueous layer to the organic layer as they create distinct layers. The solvent forms the upper layer in the funnel, and the water forms the lower layer.
By opening the stop cock and gathering the two layers in different bakers, the two layers can be restored. The solute can be retrieved after the organic solvent has evaporated.
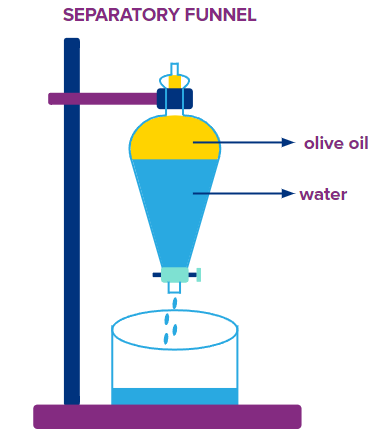
For instance, using benzene, water-soluble benzoic acid can be extracted from its aqueous solution. Finally, by distilling out the organic solvent, benzoic acid can be extracted from its solution. Two liquids like olive oil and water can be separated using this method.
Uses of Solvent Extraction:
- In the production of vegetable oil, biofuel, and other things, it is employed.
- This technique allows for the recovery and reuse of plutonium that has been radioactively irradiated from nuclear fuel.
- Hazardous pollutants are separated from sludge and sediments using it.
- It is employed in the pharmaceutical business for microsphere preparation.
- It is used to separate and purify organic molecules.
- A plentiful and steady supply of food is among humanity's most fundamental requirements. In the modern world, oilseeds, especially the soybean, are a significant source of both protein and vegetable oil. This plentiful resource is generally processed via solvent extraction, a reliable and effective method for separating the high-protein meal particles from the high-energy edible oil.
- Fruit and plant leaves are mainly made of cellulose and water, but they may also include "essential oils," a greasy mixture of substances that encapsulates the "essence" of the fragrance and flavour of the plant material. Since limonene makes up around 95% of orange oil, it may be extracted from the rind into an organic solvent like hexanes or dichloromethane owing to its nonpolar nature.
- It provides a stronger separation effect than chemical precipitation, a higher level of selectivity, and a faster mass transfer than the ion exchange approach, when compared to other separation techniques. Solvent extraction has advantages over distillation, including low energy consumption, high production capacity, quick action, simple continuous operation, and simplicity of automation. In order to adapt to the growth of DNA restriction and genetic engineering techniques, a number of innovative separation techniques have been created in recent years. These techniques include reverse micelles extraction, supercritical fluid extraction, liquid membrane extraction, and others.
Practice Problems:
Q1. By what law is solvent extraction governed?
(A) Nernst distribution law
(B) Boyle’s law
(C) Charles law
(D) Ostwald dilution law
Answer: (A)
Solution: Solvent extraction is a technique used to separate dissolved components from solutions. The "rule of disintegration of Nernst" provides the foundation for solvent extractions. The distribution of a solute between two immiscible solvents is governed by the distribution law, often known as Nernst's distribution law.
Nernst's Law of Distribution states that Only a specific ratio of a solute can be distributed across two immiscible solvents at a constant temperature. The Nernst distribution law enables us to identify favourable circumstances for extracting chemicals from solutions.
Q2. The solvent extraction technique is
(A) Qualitative
(B) Quantitative
(C) Identification
(D) None of the above
Answer: (B)
Solution: The process of transferring a substance from one solvent to another due to differences in solubility or distribution coefficient between these two immiscible (or moderately soluble) solvents is known as solvent extraction. It is a method of quantitative compound separation.
Q3. A bottle containing two immiscible liquids is given to you. They may be separated by using
(A) Fractional distillation
(B) Separating funnel
(C) Steam distillation
(D) None of the above
Answer: (B)
Solution: We should first understand what immiscible liquids are. Immiscible liquids are those that are insoluble in one another, do not mix together, and exist separately in the mixture.
Most often, in laboratories, separating funnels are used to separate mixtures of two immiscible liquids based on the difference in densities.
By gradually raising the temperatures and collecting each component individually, fractional distillation is the technique of separating mixtures based on their boiling points.
In order to reduce the boiling points of the compounds, steam water is introduced to the distillation apparatus during the steam distillation process, which separates compounds based on the difference in their temperatures.
Q4. Among the following, which is the best solvent for the extraction of oil from oilseeds?
(A) Hexane
(B) Dichloromethane
(C) Both A and B
(D) None of the above
Answer: (A)
Solution: Hydraulic pressing, expeller pressing, and solvent extraction have all been used in traditional oilseed oil extraction. Solvent extraction is one of these techniques that has seen a lot of adaptation due to practical and financial considerations and the solvent used for this extraction is hexane.
Hexane is frequently used for oil extraction because to its good solubilizing properties, narrow boiling point (336-342K) and ease of oil recovery. On the other hand, hexane is released into the environment during the extraction and recovery operations, where it reacts with the contaminants to produce ozone and phytochemicals.
Frequently Asked Questions(FAQs):
Q1. What is the separation factor?
Answer: If there are two solutes in the solution that have to be extracted, A and B, and we need to separate A from B, then we will employ an extracting solvent that will dissolve more A and very little B.
Under these circumstances, the effectiveness of separation is stated in terms of the separation coefficient or separation factor. It is the ratio of the distribution coefficients of the two solutes A and B and is represented by the symbol
To separate two solutes by solvent extraction, the separation factor needs to be very high; else, clear separation will be extremely hard.
Q2. Why does solvent extraction have more advantages than distillation?
Answer: Solvent extraction is preferable to distillation because of its low energy usage, high production capacity, quick response, simple continuous operation, and automation ease.
Q3. What is the use of solvent extraction in hydrometallurgy?
Answer: A solvent extraction process can separate and purify almost any known metal and the majority of nonmetals.
Solvent extraction is primarily used in hydrometallurgy to economically clean and concentrate mineral values from solutions. Obviously, it cannot generate a profitable recovery from an ore when the initial cost of bringing the desired element or components into solution is excessive.
Q4. Why is dichloromethane employed in the caffeine extraction process?
Answer: Extraction is a process used to remove an organic substance like caffeine from a mixture of other substances. When caffeine is extracted using a chemical solvent like dichloromethane, this process is known as a solvent-based extraction.
Since dichloromethane has a considerably higher solubility for caffeine than water. The favoured solvent for the extraction of caffeine was dichloromethane. Unroasted coffee beans are steamed after which they are repeatedly washed in dichloromethane. Before being drained away, the dichloromethane would remove the caffeine from the object. As a result, flavorful coffee beans without a kick were produced.


