-
Call Now
1800-102-2727
Sodium Chloride- History and Occurrence, Structure, Preparation, Physical and Chemical Properties, Uses
Imagine a chef in an elite-class hotel, preparing his signature dish. He might be on a spree of adding the world’s most exotic and expensive spices and ingredients to this luxurious dish, but one simple thing he missed and the dish tasted disastrous.
What could that ‘simple’ thing be? It is the ‘salt’ or our very own sodium chloride. We can’t even imagine a single day without using it. From luxurious hotels to our simplest home kitchens, it is widely and extensively used. It is the ultimate source of taste builder in our food apart from its enormous other usages.

TABLE OF CONTENTS
- History of Sodium Chloride
- Occurrence of Sodium Chloride
- Structure of Sodium Chloride
- Preparation of Sodium Chloride
- Physical Properties of Sodium Chloride
- Chemical Properties of Sodium Chloride
- Uses of Sodium Chloride
- Practice Problems
- Frequently Asked Questions
History of Sodium Chloride (NaCl)
The most significant salt deposits have originally come from the evaporation of seawater at some point of time during the past geological ages. It is roughly estimated that 78 % of the mineral content in the natural seawater is sodium chloride.
Even today, some populations of the world can't access salt, or we can say that having salt in their daily use is still a luxury for them. In some central African countries, people generally survive on roasted or raw meat and milk, but those who survive mostly on various vegetables and cereals need supplements of salt.
In Ethiopia and other regions of Africa, as well as Tibet, salt cakes were used as money. A salt allowance was granted to the officers and all the men of the Roman army; this salarium (from whence the English term salary was derived) was turned into a salt allowance in money throughout imperial times.
Some of the world’s leading producers of salt of this century are China, India, USA, Canada, and Germany.
Occurrence of Sodium Chloride
Usually, all the compounds that comprise either sodium or chlorine are derived from salts. As a result, it is found in abundance in nature. Salt is a major constituent of the dissolved mineral matter in seawater.
One way we can get pure salt is from halite mineral. The saline solution makes the mining of the deposits easy as it can create sodium chloride or NaCl solutions and therefore transport of the mineral through water from deposits is usually carried out. The salts are therefore dissolved, and the solution is subsequently pumped away.
Another wat is the saltwater evaporation which is one of the most frequent methods for salt production and is used in many countries, including India. But the salt obtain in crystals mostly has certain impurities like sodium sulphate, calcium sulphate, etc. if we want to get a pure crystal of sodium chloride, we dissolve the crystals in water and then filter it using a filtration method.
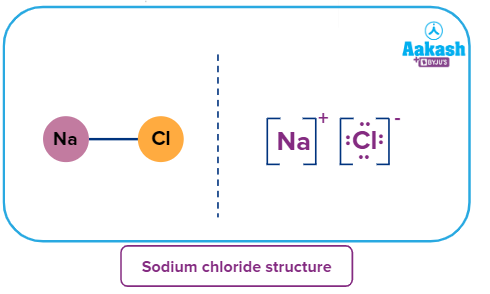
Structure of Sodium Chloride
Sodium chloride is an ionic solid. It is composed of electrovalent bonds between Na+ and Cl-ions.
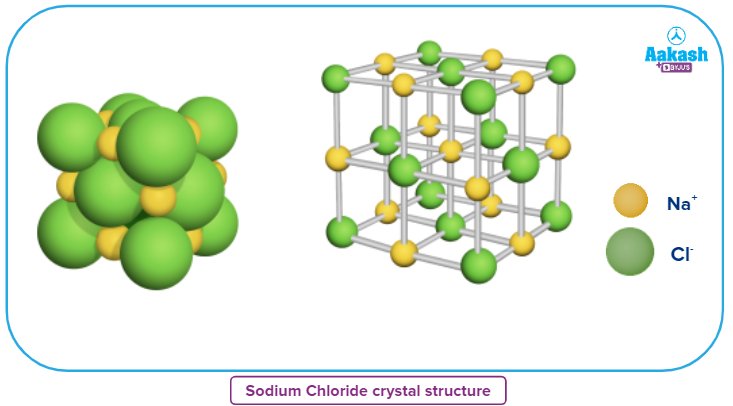
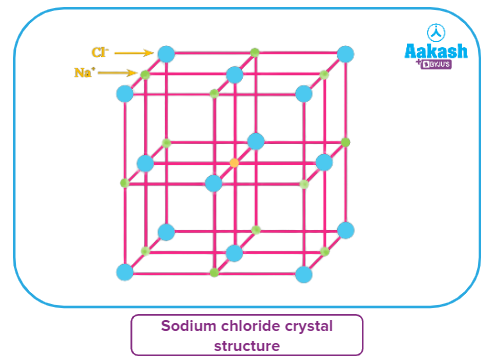
The crystal lattice of NaCl (rock salt structure) has a face-centered cubic arrangement of anions (Cl-) and cations (Na+ ) occupy all the octahedral voids. Each ion in the unit cell has a coordination number of 6.
Recommended video: https://www.youtube.com/watch?v=IvhLM5wuiI4&t=5s
Preparation of Sodium Chloride (NaCl)
Evaporation of seawater
The most abundant source of sodium chloride is seawater, which contains 2.7 to 2.9% of salt by mass. Sea salt is the term used for the salt obtained by the evaporation of seawater. It is used for seasoning food, cooking, making different cosmetics and food preservation. It is also called bay salt, solar salt, or simply salt.

Crude NaCl (from evaporation) contains sodium sulphate, calcium sulphate, calcium chloride, and magnesium chloride as impurities. Calcium chloride and magnesium chloride are soluble impurities because they are deliquescent.
Now to purify crude salt, it is dissolved in a minimum amount of water and filtered to remove insoluble impurities. The solution is then saturated with HCl gas to give crystals of pure sodium chloride. Calcium chloride and magnesium chloride being more soluble than NaCl, remain in the solution. Insoluble impurities from crude salt are removed through the process of filtration.
Laboratory preparation of NaCl
In laboratories, we can combine sodium and chlorine to form what is known to be one of the most common substances, i.e., sodium chloride.
Preparation of sodium chloride from Acid-Base Reaction
Sodium hydroxide reacts with hydrochloric acid to produce sodium chloride and water. This is an example of a neutralisation reaction.
![]()
Physical properties of Sodium Chloride (NaCl)
- NaCl is an ionic compound with high boiling and melting points i.e., 1465 °C and 801 °C respectively.
- Molar mass of sodium chloride is 58.44 g mol-1.
- Density of NaCl is 2.165 g cm-3.
- It is a crystalline white solid which is highly soluble in water and its water solution is called saline. But it is only partially soluble or immiscible in other organic solvents.
- It has a solubility of 36.0 g in 100 g of water at 273 K.
- The aqueous and molten state of sodium chloride is an excellent conductor of electricity as the structure allows the free and easy movement of ions.
Chemical properties of Sodium Chloride (NaCl)
- Sodium chloride on flame test gives golden yellow colour corresponding to the Na+ ions.
- It is a neutral salt.
- Sodium chloride can undergo a double displacement reaction with salts like AgNO3.
![]()
- Sodium chloride reacts with dilute sulphuric acid to form sodium sulphate and hydrochloric acid upon being heated.
![]()
- On electrolysing a brine solution (mixture of sodium chloride and water), aqueous sodium hydroxide is obtained along with the evolution of hydrogen and chlorine gas.
![]()
- Sodium chloride is used in Solvay’s process to produce sodium carbonate with the help of the following reactions:
![]()


- Sodium chloride does not react with hydrogen peroxide as it is a very stable salt.
Uses of Sodium Chloride
- Sodium chloride has a lot of applications in various industries like food, pharmaceuticals etc.
- It acts as a food preservative as well as a seasoning for food.
- In the production of sodium hydrogen carbonate and sodium carbonate, sodium chloride is a very important raw material.
- Sodium chloride is also used for the production of glass.
- It is used in making various medicinal products like ORS (oral rehydration solution), saline drops for eyes, saline water in hospitals etc.
- It is used in fire extinguishers.
- It is used in cleansers like shampoo and toothpaste.
- It is used in the paper industry, textile industry and in the construction of roads.
- It is used in water softening.
Practice Problems
Q.1. What is the observation when a sample of sodium chloride is made to undergo a flame test?
A. It explodes
B. It gives off a yellow gas
C. It burns with a golden yellow flame
D. No observation
Answer: (C)
Solution:
Sodium ions give a characteristic golden-yellow flame. It is an alkali metal with low ionization enthalpy. Hence, it readily ionises and produces a characteristic golden yellow coloured flame.
Q.2. What type of bonding is present in sodium chloride?
A. Coordinate Bonding
B. Covalent Bonding
C. Electrovalent Bonding
D. Hydrogen Bonding
Answer: (C)
Solution:
Sodium chloride structure constitutes Na+ and Cl-. They have the strong electrostatic force of attraction between them. This is why ionic bonds or electrovalent bonds are present in sodium chloride.
Q.3. What kind of crystal structure does rock salt possess?
A. BCC
B. FCC
C. HCP
D. CCP
Answer: (B)
Solution:
The crystal lattice of NaCl (rock salt structure) has a face-centered cubic arrangement of anions (Cl-) and cations (Na+ ) occupy all the octahedral voids.
Q.4. The pH of sodium chloride solution is:
A. 7
B. 9
C. 13
D. 0
Answer:
Solution:
Since sodium chloride is a neutral salt made of 1:1 of sodium and chloride ions. Hence it has a pH of 7.
Frequently Asked Questions-FAQs
Q.1. Why is NaCl necessary for our body?
Answer: NaCl is necessary for our body because of the following reasons:
- It helps to maintain normal blood pressure.
- It assists in maintaining the fluid balance of our body.
- It has various biological significance like nerve-signal transmission and muscle relaxation.
Q.2. Why does common salt become wet in the rainy season?
Answer: This is due to the presence of some traces of impurities like MgCl2and CaCl2in common salt besides NaCl. MgCl2and CaCl2 are highly hygroscopic in nature and absorbs moisture from the air especially during humid climate of the rainy season. Therefore, common salt tends to get wet.
Q.3. Why is sodium chloride given to patients?
Answer: Around 23.4% of NaCl solution can be used to treat a low-salt syndrome in our body as it helps to replenish the water and salt. It can be also added as an additive in TPN (total parenteral nutrition) and in intravenous fluids (IV) containing carbohydrates (saline water). Also, it is used to prepare saline water for treating nasal blockages during cough and cold.
Q.4. What is the side effect of a medical overdose of sodium chloride?
Answer: An excessive overdose of NaCl can lead to faster heartbeats, shortness of breath, trouble swallowing and swelling of feet, hands, face or eyelids.


