-
Call Now
1800-102-2727
Sodium Carbonate Bead Test- Qualitative Analysis for Dry Test, Borax, Borax Bead Test, Practice Problems, FAQs
You must have seen different coloured salt present in your chemistry labs with labels marked on the bottles. Imagine if the labels would not be there, how can one guess the nature of the salts?
It would be difficult for us to remember all the colours and names of the compounds so we label them.
Let us suppose your teacher gives you four salt of different colour and ask you to name them.
For this, you will perform certain tests as per the nature of your salt. This will confirm the names.
Today, we will be discussing a dry test name sodium carbonate bead test which is almost similar to the famous borax bead. Preliminary investigation of salt frequently yields useful information that streamlines the subsequent course of analysis. Although these tests are not conclusive, they can provide vital information about the presence of specific anions or cations. These exams can be completed in as little as 10-15 minutes. These include taking note of the salt's overall look and physical attributes, such as colour, smell, and solubility. Dry tests are what they're called.
Let us study in detail, how these tests are performed.

Table of content:
- Qualitative analysis for dry test.
- Borax
- Borax bead test
- Sodium carbonate bead test
- Practice problems
- Frequently asked questions. FAQs
Qualitative analysis for dry tests:
- The process of finding the nature of a substance and the identity of its constituents is known as qualitative analysis.
- Qualitative analysis of inorganic salts means the identification of cations and anions present in the salts.
- Qualitative analysis is carried out through reactions that are easily detectable to human senses such as sight and smell.
- A dry test involves noting the appearance and physical properties, such as color, odour, solubility, etc. of the salt.
- E.g- Heating of dry salt, flame tests, borax bead test, microcosmic salt bead test, charcoal cavity test, etc.
Borax:
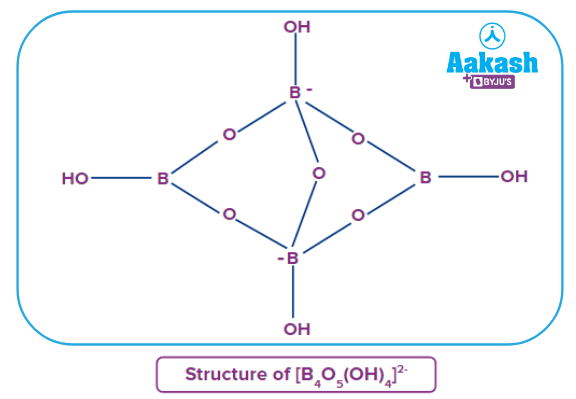
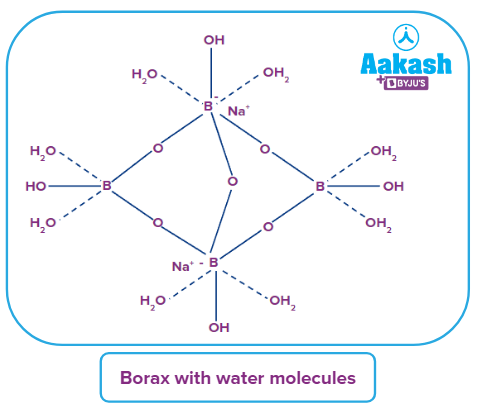
Borax is a compound consisting of boron, united with oxygen and sodium. It contains the tetranuclear units. The formula of borax is . Borax dissolves in water to give an alkaline solution. It contains five B−O−B linkages.
Borax-Preparation:
- When colemanite powder is heated with sodium carbonate solution, calcium carbonate is precipitated.
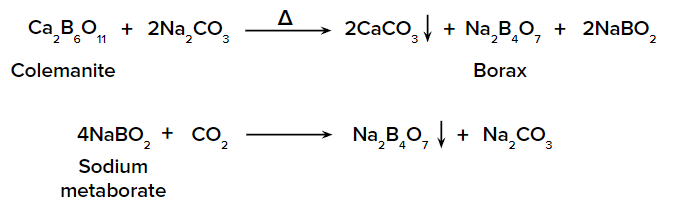
The solution is now concentrated and cooled to crystallise borax. is orthoborate and is metaborate.
- By the action of Na2CO3 on H3BO3.
4H3BO3 + Na2CO3 --> Na2B4O7 + 6H2O + CO2
Borax-Bead test:
- The bead test is a classic qualitative inorganic analytical method for detecting the presence of specific metals. The borax bead test, often known as a blister test, is the earliest. Berzelius was the first to introduce it in 1812.
- The mixture of sodium metaborate and boric anhydride as obtained on heating borax as explained above forms borax beads.
- Borax reacts with certain metal salts such as etc., to form coloured metaborates. The colour of the metaborates can be used to identify the metallic ions (cations) in its salts. This is called borax-bead test. The bead test isn't a foolproof way to identify an unknown metal, but it might help you rule out or narrow down your options quickly.
Example:
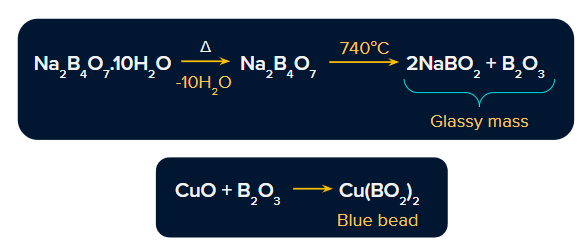
Formation of copper beads: First, copper salt is heated to form copper oxide.
On further heating with borax beads, this copper oxide forms copper metaborate, which has a sky blue colour.
![]()
|
Colour |
Oxidising |
Reducing |
|
Colourless |
hc:Al, Si, Bi, Sn, Cd, Mo,Pb,V,Ti,W |
Al, Si, Sn Alkaline earth metals, |
|
Opaque/grey |
sprs: Al, Si, Sn |
Ag, Bi, Ni,Cd, Zn,Pb,Sb |
|
Green |
Cr, Cu |
Cr |
|
Blue |
c: Cu |
hc:Co |
|
Red |
c:Ni |
c:Cu |
|
Violet |
Ni+Co |
c:Ti |
|
Yellow /Brown |
h, ns: Fe,V, U |
W |
The keywords used here in the above table are
h=hot
c=cold
hc=hot or cold
s=saturated
ns=not saturated
sprs=supersaturated
Sodium carbonate bead test:
- The sodium carbonate bead test is an analytical procedure that involves reducing the metal's oxides or sulphides to the metallic form using sodium carbonate.
- When these oxides are subjected to flame, they produce distinct colours. As a result, it aids in the identification of the metal present.
- The technique involves mixing the finely powdered metal to be identified with sodium carbonate (which produces the bead) and charcoal, with the finely powdered metal accounting for only one-third of the overall combination.
- The mixture is then moistened and placed in the hollow of the charcoal, where it is heated over the flame. As a result, the colour of the flame is monitored and compared to the available bead type and colour data.
- Sodium chromate is formed when chromium oxide,Cr2O3, reacts with sodium carbonate. The chromium oxide has chromium in the (+3) oxidation state, which is green in colour and is reduced to the (+6) oxidation state in sodium chromate, which is yellow in colour.
The colour of the bead in the sodium carbonate bead test with Cr2O3 is yellow.
Practice Problems:
Q1. How much water of crystallisation is present in borax?
A. 3
B. 8
C. 7
D. 5
Answer: B
Solution: Water of crystallisation means having a fixed number of water molecules in one formula unit
of salt. Crystal salts with water of crystallisation are known as hydrates. The other names for the water of crystallisation are crystallisation water or water of hydration. The correct formula for borax is . Therefore, borax has 8 molecules of water of crystallisation.
Q 2. The colour shown by cobalt during the borax bead test is
A. Green
B. Purple
C. Blue
D. Olive
Answer: (C)
Solution: Blue colour Co(BO2)2 (cobalt metaborate) formation takes place. On treatment with metal salts, the below-mentioned reaction takes place.
Q3.The glassy bead forms a coloured metaborate in_____________flame when heated with a colored salt.
A. Reducing flame
B. Oxidising flame
C. Cool flame
D. Thermonuclear flame
Answer: (B)
Solution: The glassy bead forms a coloured metaborate in Oxidising flame when heated with a coloured salt. Below is the mentioned reaction, to this.
Cu(BO2)2 is a blue-coloured salt formed in the oxidising flame.
Q4. During this bead test,the bead is dipped in the coloured salt after being moistened with__________.
A. alcohol
B. water
C. ether
D. acetone
Answer: B
Solution: The bead is dipped in the coloured salt after being moistened with water. It is now heated in both hot and cold circumstances, first in an oxidising flame and subsequently in a reducing flame, with colours visible in both flames.
Frequently asked questions- FAQ
Q1. What is a microcosmic bead test? Mention the colour changes in reducing and oxidising flames.
Answer: This test is used for coloured salts because microcosmic salts react with corresponding transition metal salts to form metal orthophosphate and show their characteristic colors. Same procedure as a borax bead test.colour changes in reducing and oxidising flames for important metals are mentioned below:
|
Oxidizing flame |
Reducing flame |
Metal |
||
|
Hot |
Cold |
Hot |
Cold |
|
|
Green |
Blue |
Colorless |
Red |
Copper |
|
Brown |
yellow |
Green |
Green |
iron |
|
Blue |
Blue |
Blue |
Blue |
Cobalt |
|
Violet |
Brown |
grey |
grey |
Nickel |
Q2. What are flame tests? Mention the colour observed for important metals.
Solution: The non-luminous flame is coloured by certain volatile salts. Compared to other salts, metal chlorides are more volatile. The metal chloride is vaporised and thermally ionised.
Because cations absorb energy from the flame and transmit it as the light of a specific colour, they give the flame a distinct colour.
The wire's tip is strongly heated in a non-luminous flame, and the flame's colour may be seen with the human eye.
|
S.No |
Color of the flame |
Inference |
|
1. 2. 3. 4. 5. 6. |
Golden yellowViolet Brick red Crimson red Apple green Green with a blue cenre |
Sodium Potassium Calcium Strontium Barium Copper |
Q3. What salt fails the borax bead test?
Solution: Only coloured metals pass the borax-bead test, which is used to identify transition metals, while colourless ions do not.Other metals do not give this test because colourless ions do not perform this test.
Q4.What are the properties of borax?
Solution: Borax is a white crystalline solid.
- It is less soluble in cold water but more soluble in hot water.
- Its aqueous solution is alkaline.
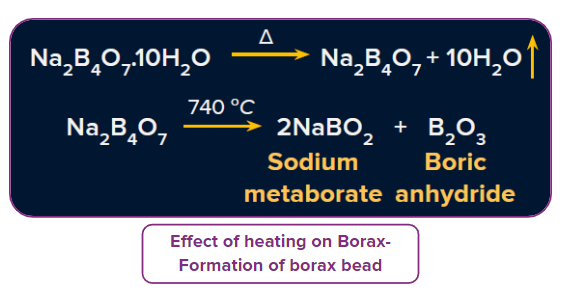
- Effect of heating: It first swells due to loss of water in the form of steam. When borax powder is heated, it first swells due to the loss of water in the form of steam. However, at 7400C, it gets converted into colourless transparent borax bead.