
Second Order Reaction - Definition, Formula, Differential Integrated Rate Equation, Graph and Half-Life of Second-Order Reactions
The reactions in which the sum of exponents in the corresponding rate law of the chemical reaction is equal to two are known as second-order reactions. The rate of such reactions can be written either as
r = k [A] 2, or as r = k [A] [B].
From the above equation, we know that second-order reactions are those chemical reactions that either depend upon the concentrations of two first-order reactants or on the concentration of one second-order reactant.
These two types of second-order reactions can be described as follows-

The sum of x and y (which corresponds to the order of the chemical reaction in question) equals two.
Examples of Second-Order Reactions
Examples of second-order reactions include the following reactions-
1. NH4CNO → H2NCONH2
Ammonium cyanate in water isomerized into urea.
2. H+ + OH- → H2OC + O2 → CO +O
The two examples given above are the second-order reactions depending on the concentration of two separate first order reactants.
3. CH3COOC2H5 + NaOH → CH3COONa + C2H5OH
Here, an example of the hydrolysis of an ester in the presence of a base, ethyl acetate in the presence of sodium hydroxide.
2NO2 → 2NO + O2
4. 2HI → I2 + H2
These reactions involve one second order reactant yielding the product.
5. 2 NOBr → 2 NO + Br2
In the gas phase, nitrosyl bromide decomposes into nitrogen oxide and bromine gas.
6. H+ + OH- → H2O
Hydrogen ions and hydroxyl ions form water.
7. 2 NO2 → 2 NO + O2
Nitrogen dioxide decomposes into nitrogen monoxide and an oxygen molecule.
8. 2 HI → I2 + H2
Hydrogen Iodide decomposes into iodine gas and hydrogen gas.
9. + O3 → O2 + O2
During combustion, oxygen atoms and ozone can form oxygen molecules.
10. O2 + C → O + CO
Another combustion reaction, oxygen molecules react with carbon to form oxygen atoms and carbon monoxide.
11. O2 + CO → O + CO2
This reaction often follows the previous reaction. Oxygen molecules react with carbon monoxide to form carbon dioxide and oxygen atoms.
12. + H2O → 2 OH
One common product of combustion is water. This can react with all the loose oxygen atoms produced in the previous reactions to form hydroxides.
Differential and Integrated Rate Equation for Second-Order Reactions
The differential rate law equation considering one second order reactant results a product in chemical reaction can be written as
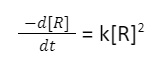
In order to get the integrated rate equation, this differential form must be rearranged:

Integrating both the sides considering with the change in the concentration of reactant between time 0 and time t, we get the following equation-
|

From the power rule of integration, we have:

Where C is the constant of Integration. Now, using this power rule in the previous equation, we get the following equation as-

This is the required integrated rate expression of second order reactions.
Graph of a Second Order Reaction
We need to generalize [R]t as [R] and rearrange the integrated rate law equation of reactions of the second order to get the following reaction-

Plotting a straight line (y=mx + c) corresponding to this equation (y = 1/[R] , x = t , m = k , c = 1/[R]0)
We can observe the slope of the straight line is equal to the value of the rate constant, k.
Half-Life of Second-Order Reactions
The time taken for half of the initial amount of reactant to undergo the reaction is known as the half-life of the second-order reaction.
The following substitutions should be done to obtain the desired equation-
[R] = [R]02
And, t = t1 / 2
Substituting these values in the integral form of the rate equation of second order reactions, we get:

Therefore, we get the required equation for the half-life of second order reactions-

We can conclude from the above equation that the equation for the half-life implies that the half-life is inversely proportional to the concentration of the reactants.
Key Points
- A second-order reaction will depend on one second-order reactant's concentration(s) or two first-order reactants.
- The method of initial rates is used to determine the order of a reaction concerning each reactant.
- When applying the method of initial rates to a reaction involving two reactants, it is necessary to conduct two trials in which the concentration of the first compound is held constant and the concentration of the second compound changes. The other two trials must also be conducted in which the concentration of the second compound is held constant, and the concentration of the first compound changes.
NEET Related Links
JEE MAIN Related Links
JEE Main marks vs rank vs percentile
JEE Advanced Related Links
JEE Advanced Eligibility Criteria
JEE Advanced Chemistry Syllabus
JEE Advanced Registration Dates

