-
Call Now
1800-102-2727
Salt Bridge: Function, Types of Sat Bridge, Working, Importance, Practice Problems and FAQs
Imagine you have two younger siblings who are absolutely naughty and always up to annoy each other. Due to an official function, your parents were out of town and will be coming back home in the evening. They have given the following instructions to you:
- Make sure your siblings don’t fight with each other.
- They should finish their lunch on time.
- Ice Cream should be distributed equally.
- Most importantly, they should not start watching cartoons until they finish their homework.

Can you manage the given responsibilities?
I can understand that’s a whole lot of responsibilities loaded on you. Well, a similar kind of responsibility is taken care of by a salt bridge in an electrochemical cell so that the respective cell can perform its work efficiently. Let’s understand the concept of a salt bridge, its function and its importance.
Table of Contents
- Definition of Salt Bridge
- Function of Salt Bridge
- Types of Salt Bridge
- Preparation of a Salt Bridge
- Working of a Salt Bridge in a Galvanic Cell
- Representation of Salt Bridge in Cell Representation
- Importance of Salt Bridge in a Galvanic cell
- Practice Problems
- Frequently Asked Questions (FAQs)
Definition of Salt Bridge
In an electrochemical cell, a salt bridge is a component that joins the oxidation and reduction half cells. A salt bridge is a junction that joins the anode and cathode electrodes compartments.
Salt bridge is generally an inverted U-shaped tube. Inside this tube, there’s a concentrated solution of inert electrolytes such as potassium chloride (KCI), potassium nitrate (KNO3), potassium sulphate (K2SO4), etc. or the solid form of these inert electrolytes in agar-agar and gelatin.

Function of Salt Bridge
A salt bridge's primary job is to assist in preserving the electrical neutrality of the internal circuit. Additionally, it helps the cell by providing aid in such a way that cell reactions won’t cease or reach equilibrium. If the cell reactions are stopped, no electricity will be generated. This can be understood by taking the example of a Cu-Zn galvanic cell.
This galvanic cell is made up of two beakers containing Zinc and Copper rods. These rods are dipped inside their respective salt solutions. (ZnSO4, CuSO4). The redox reactions continue in both the half cells, after some time one electrode will start accumulating Zn2+ions, whereas the other electrode will start depositing ions. Ultimately a stage will reach where all ions will be consumed and the reactions will stop. Hence, to avoid this and to maintain electroneutrality, a salt bridge containing KNO3 is used.
As a result, a salt bridge essentially aids in preventing the buildup of positive and negative charges around the corresponding electrodes while also facilitating a smooth reaction. Additionally, it facilitates the steady flow of electrons. However, since the electrons are moving from one half of the cell to the other, the goal of a salt bridge isn't to transfer electrons from the electrolyte but rather to maintain charge equilibrium. Two half cells are connected electrically by a salt bridge.
The main purpose of a salt bridge is to preserve electrical neutrality between two half cells. To achieve this, an inert electrolyte must be used. The two half cells require the ions to move back and forth between them. Compared to other salts, potassium nitrate (KNO3) and potassium chloride (KCl) are more effective inert salts. To stop the reactions between the salt and solution, an inert electrolyte is used. Because potassium and chloride ions have a very similar size which makes the mobility of both cationic and anionic parts equivalent, the inert salt potassium chloride (KCl) is frequently used. However, potassium chloride shouldn't be used as an electrolyte when using a lead or silver electrode because it will precipitate.
Types of Salt Bridge
In electrochemical cells, salt bridges primarily come in two varieties:
- Salt Bridge with glass tube (Glass tube bridge)
- Paper filter bridge
Glass tube bridge
Typically, they are electrolyte-filled U-shaped tubes. Common electrolytes include sodium chloride (NaCl), potassium chloride (KCl), and potassium nitrate (KNO3). The cell's other chemicals must not react with the electrolyte very much, and its cations and anions must migrate at roughly the same rate.
Most frequently, gels like Agar-Agar are used to hold the electrolytes. Conductivity is significantly influenced by the glass tube's diameter and salt solution concentration. Reduced concentration and tube diameter result in a reduction of conductivity.
Filter paper bridge
Another widely used salt bridge is the filter paper bridge. They are made of filter paper or another porous material that has been saturated in an electrolyte. Electrolytes like potassium chloride (KCl) or sodium chloride (NaCl) are frequently used in this type of salt bridge. The conductivity of filter paper is influenced by its porosity, roughness, and electrolytic concentration. Smooth absorbent filter papers are used for higher conductivity because they produce a higher conductivity than rough papers with lower absorbents.
Preparation of a Salt Bridge
Putting string, cotton, or paper in an electrolyte solution to soak
Take material that can reach two beakers in order to prepare a bridge. Place this material in an electrolyte pool until it is completely saturated. Take the material out of the electrolytic solution with care, then drain off the extra electrolyte.
Making Gel That Will Serve as a Bridge
The gel is treated with a buffered solution and heated after being suspended in an electrolytic solution. On a glass plate or tube, the viscous gel is allowed to set.
Working of a Salt Bridge in a Galvanic Cell
Let’s consider a salt Cu-Ag galvanic cell;
The reaction is:
Above reaction can be written as:
The two half-reactions will be:
(Oxidation half-reaction)
(Reduction half-reaction)
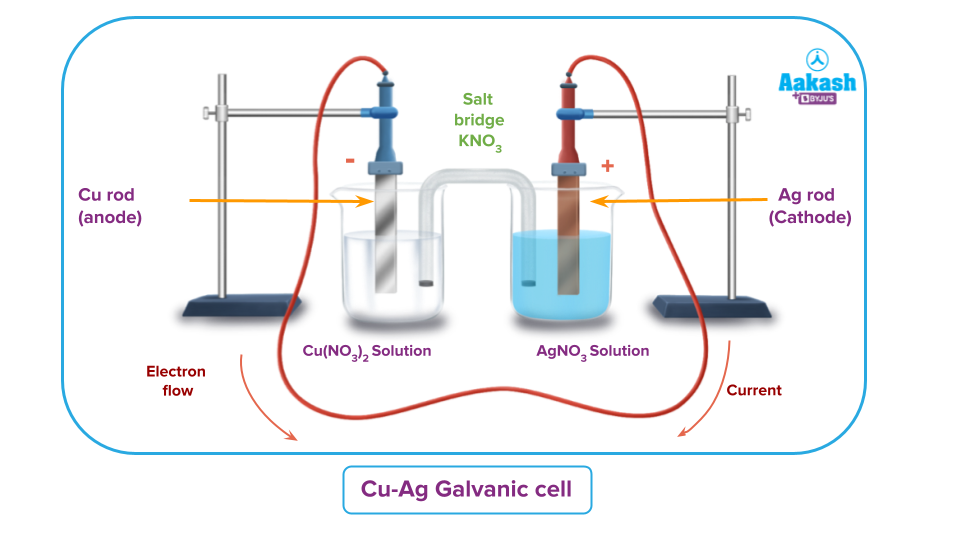
Positive ions and electrons are produced at the anode where oxidation reaction is taking place. Now, after some time, a few unbalanced positive charges are left in the anode compartment as the electrons pass through the wire. The negatively charged () ions from the salt bridge move towards the anode compartments to maintain electrical neutrality.
Similarly, as Ag+ ions in the cathode compartment are getting used up from the solution. There will be accumulation of ions after some time. So to maintain the electrical neutrality K+ ions move from the salt bridge towards the cathode compartment.
Consequently, the salt bridge is used to maintain the electrical neutrality of the solution.
Representation of Salt Bridge in Cell Representation
- By convention, the electrode on which oxidation takes place (oxidation half cell) is written on the left-hand side and the other electrode on which reduction takes place is written on the right-hand side.
- The oxidation half cell on the left-hand side is written in order starting with the symbol of the metal (or the gas) first, followed by the symbol of the ion in equilibrium with its concentration in brackets. The reduction half cell is written in the reverse order of the ion in equilibrium with a concentration in the bracket followed by the symbol of the cathode metal (or the gas).
- Single vertical lines separate the metal electrode and the ion in equilibrium for both the half cells.
- The two half cell is separated by a double vertical line and indirectly indicates the presence of a connecting salt bridge connecting the two electrolytes.
Metal | Metal ion (conc.) || Metal Ion (conc.) | Metal
Anode (Oxidation) Salt bridge Cathode (Reduction)
According to the above rule we can represent the Cu-Ag galvanic cell as:
Where C1 and C2 are the concentration of the respective ions and || symbol represents the salt bridge between the two compartments.
Importance of Salt Bridge in a Galvanic cell
Electricity is produced by a redox reaction in a galvanic cell. In a galvanic cell, a salt bridge is significant. Let's examine what unfolds if the galvanic cell's salt bridge is absent.
- Each beaker contains a neutral solution before the wires are connected. Consequently, the positive and negative charges are equal in number. Two electrons will be lost by the zinc bar, which serves as the galvanic cell's anode. so that the wire allows the electrons to travel to the copper bar, which serves as the galvanic cell's cathode.
- As a result of zinc losing electrons, the solution now has a positive charge. One positive copper ion (Cu2+) leaves the solution and accepts the two electrons when the copper bar (cathode) absorbs the two electrons.
- A copper atom will then deposit on the copper bar as a result of this. As a result, this solution acquires a negative charge.
Currently, there are two voltages present: one between the electrodes (a metal bar), and the other between the charged solutions. Positive voltage exists between the metal bar or electrode and negative voltage exists between the charged solution. Due to the cancellation of these voltages, no current will flow. This demonstrates the critical role salt bridges play in electrolysis and all electrochemical cells.
Recommended Video: Salt Bridge
Time Stamp: 22:39 to 25:33
Practice Problems
Q.1 Salt bridge involves the transfer of_____
A. Electrons
B. Holes
C. Ions
D. Charges
Answer: (C)
Solution: The flow of ions between the oxidation and reduction half-cells is facilitated by a salt bridge to maintain the electrical neutrality and to provide aid for cell reaction not to cease.
Q.2 What will be the consequences if the salt bridge is removed between the half cell of a galvanic cell?
A. Voltage remains constant
B. Voltage increases gradually
C. Voltage increases rapidly
D. Voltage decreases to zero
Answer: (D)
Solution: Salt bridge completes the circuit, allowing current to flow. If the salt bridge is taken away, no current will flow and the voltage will fall to zero. Moreover, it helps in maintaining electrical neutrality.
Q.3 The salt bridge uses KNO3 because:
A. With agar-agar, it doesn’t form a jelly-like substance.
B. Almost equal amounts of K+ and ions are transported.
C. This electrolyte is weak.
D. It is not a good electrical conductor.
Answer: (B)
Solution: The salt bridge uses KNO3 because it produces K+ and ions which are transported to cathode and anode compartments in equal amounts to maintain electrical neutrality. Both of these ions have nearly the same ionic mobility.
KNO3 is a strong electrolyte and conducts electricity in a molten state. This electrolyte generally forms a jelly-like substance when it is exposed to agar-agar. Hence, options A, C, and D are incorrect.
Q.4 Find out the wrong statement with respect to a salt bridge.
A. Salt bridge should be made up of mobile cations and anions.
B. Salt bridge should be made up of static charges.
C. Ions furnished from salt bridges must not undergo oxidation or reduction at the electrodes.
D. Ions furnished from salt bridges should not interact with the ions of solutions in the cathode and anode compartments.
Answer: (B)
Solution: Salt bridge is an important component of a galvanic cell and while choosing a salt bridge certain things need to be followed such as:
- Chosen salt bridges should be made up of mobile cations and anions.
- Salt bridges should not interact with the solution's ions.
- Salt bridges must not undergo oxidation or reduction at the electrodes.
If ions are not mobile then they can’t produce electricity.
Hence, option (B) is the correct choice for this question.
Frequently Asked Questions (FAQs)
Q.1 Why is a salt bridge made with a strong electrolyte?
Answer: A connection between a galvanic cell's oxidation and reduction half-cells that contains a weak electrolyte is known as a salt bridge (e.g., voltaic cell, Daniell cell). The main purpose of the electrolyte in the salt bridge is to produce enough ions which can replace the ions lost in the half cells. In this way it maintains electroneutrality. Hence, strong electrolytes are preferred such as KNO3 as they can disscociates their ions completely in aqueous form.
Q.2 Why NaCl is not used as an electrolyte in the salt bridge?
Answer: The electrolyte in the salt bridge should have enough ions and the ionic mobility of cations and anions should be the same. Sodium chloride is a strong electrolyte and is a good conductor of electricity in a molten state or in an aqueous state. But if you compare the size of cation that is Na+ and the size of anion that is Cl-, you will observe that they have huge size differences. The size of ions is inversely proportional to the mobility of ions. Both anions and cations will have different ionic mobility. Hence, we can’t use NaCl as an electrolyte for a salt bridge.
Q.3 Why do we use agar-agar with electrolyte in the salt bridge?
Answer: Agar-agar is employed with electrolyte in a salt bridge. Because it is a non-electrolyte substance that does not break down into ions. Therefore, no ions are available for migration. Due to its gel-like composition, it inhibits any potential mixing of the two fluids that are present on the two sides of the salt bridge.
Q.4 Why does an electrolytic cell not employ a salt bridge?
Answer: Salt bridge is generally used to maintain the electroneutrality of two-half cells and helps cell reactions to continue. Electrolytic cells don't require a salt bridge because they only use one type of ionic solution where both anode and cathode are dipped in the same electrolytic solution.


