-
Call Now
1800-102-2727
Reduction of oximes, azides and nitriles: Definition of Reduction, Reducting Agents, Amines, Classification of Amines, Nomenclature of Amines, Preparation of Amines from the Reduction of Nitriles, Azides and Oximes, Practice Problems and FAQs:
Imagine that next month is your mother’s birthday. You are very excited to celebrate her birthday because recently you won a scholarship and you want to surprise her. You invited all your dear friends and family members to join the party. But now you have to arrange that party. I know it is very difficult as you haven’t done it before.
What will you do now?
Well, you can either try by yourself which is very risky at this moment or you can hire someone who can take all your responsibilities on his own and arrange a good party.
This is very common practice, if we are not good at something we can definitely ask someone else to help.
Similarly during the synthesis of organic compounds, sometimes it is difficult for the reactants to get itself reduced or oxidized hence they require an reducing or oxidizing agent which can help them to get reduced.
Let’s understand how we can prepare amines by reducing oximes, azides and nitriles.
Table of content
- Definition of reduction
- Reducing agents
- Amines
- Classification of amines
- Nomenclature of amines
- Preparation of amine from the reduction of nitriles
- Preparation of amine from the reduction of azides
- Preparation of amines from the reduction of oximes
- Practice problems
- Frequently asked questions (FAQs)
Definition of reduction
The chemical processes in which electrons are transferred from one chemical to another. Redox reactions, also known as oxidation-reduction reactions, are the name given to these electron-transfer processes.
Energy changes in the form of heat, light, electricity, etc. accompany these reactions. The addition of oxygen or hydrogen to various compounds is another step in the oxidation and reduction reactions.
- Reduction is a process that involves the addition of hydrogen or any other electropositive element or the removal of oxygen or any other electronegative element, according to classical or older concepts.
- The process through which an atom or an ion gains one or more electrons is known as reduction, according to the electronic idea.
Reducing agents
One of the reactants of an oxidation-reduction process is a reducing agent, which decreases the oxidation state of the other reactant by discharging electrons into it. The reduction process cannot take place if the reducing agent does not transfer electrons to another component during a reaction.
Some common reducing agents used in organic chemistry are given below.
|
Reducing agents |
|
Pd/Pt/Ni(H2) gas |
|
LiAlH4 |
|
NaBH4 |
|
Na/C2H5OH |
|
NaHg/C2H5OH |
|
SnCl2/HCl |
|
Sn/HCl |
|
Fe/HCl |
|
Zn/HCl |
|
Renny Ni |
Amines
Chemically speaking, amine is a product of ammonia (NH3). In other terms, amines are derived from ammonia. A nitrogen atom with a lone pair is present in amines, which are organic nitrogen molecules. Aryl or alkyl groups often replace the hydrogen atoms of ammonia in amines.
Classification of amines
Depending on how many hydrogen atoms in ammonia are swapped out for an alkyl or aryl group, amines are divided into three categories: primary (10), secondary (20), and tertiary (30). Amines of the formula R-NH2 or primary amines (10) are produced when just one hydrogen atom is changed. Secondary amines are created when alkyl/aryl groups take the place of two of the three hydrogen atoms. Tertiary amines are produced if all three hydrogen atoms are swapped out for an alkyl/aryl group.
Nomenclature of amines
According to the rules outlined by IUPAC for the nomenclature of organic compounds, the names of compounds that are universally recognised in organic chemistry are given. Aliphatic amine names take the form of alkylamine because the alkyl group is added before the amine in the naming process. For illustration, CH3NH2is known as methylamine because it combines an amine and an methyl alkyl group. When there are two or more similar groups, prefixes like di and tri are added before the names of the alkyl groups. The parent chain and the positions of the amino groups in the amine are recognised if there are many amino groups present by numbering the carbon atoms in the parent chain.
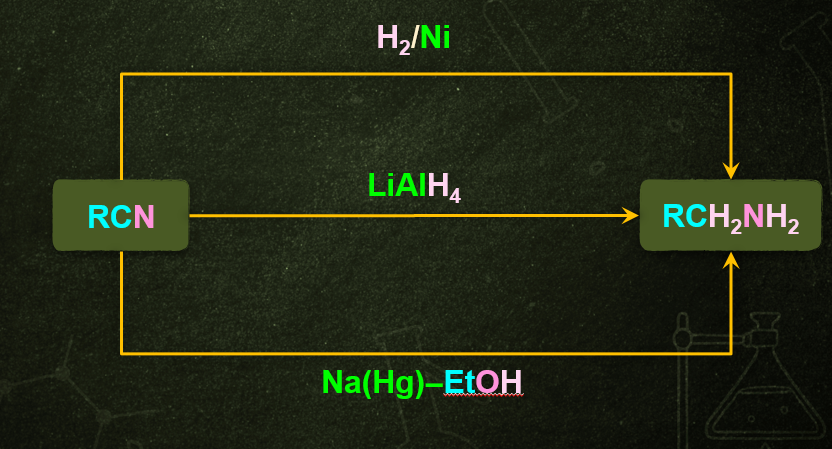
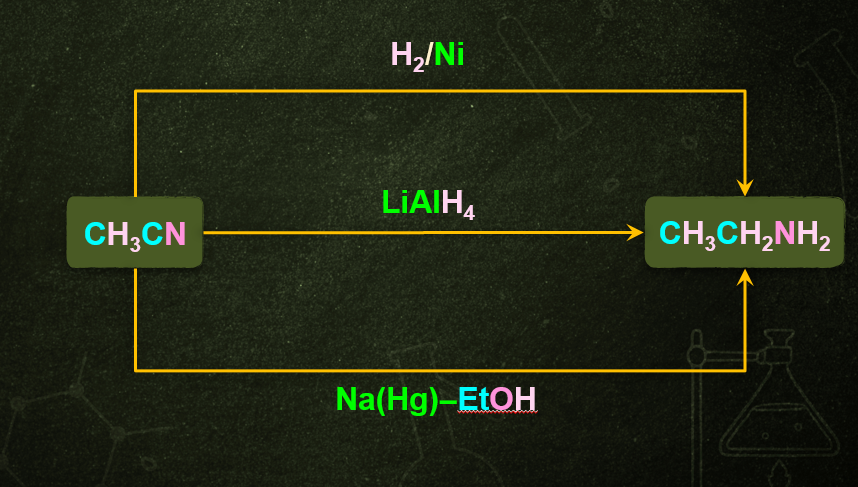
Preparation of amine from the reduction of nitriles
- In organic chemistry, nitriles, commonly known as cyano compounds, are organic molecules. The carbon atom is connected to a functional group known as the cyano group, which is denoted by the functional group (-C N).
- By reducing with lithium aluminum hydride nitriles can be converted to several primary amines.
- Nitriles which are obtained from aldehydes undergo partial reduction to form imine, this reaction is commonly known as Stephen reduction. Imine on further reduction converts into primary amine in the presence of reducing agents that is Sn/HCl.
- This reaction is used for the preparation of amines containing one carbon atom more than the starting compound.
CH3CN+4[H]CH3CH2NH2


Preparation of amine from the reduction of azides
- Any chemical compound in the class of azides that has three nitrogen atoms arranged in a group is symbolized by the symbol (-N3). Azides are thought to be generated from hydrazoic acid (HN3), a salt like sodium azide (NaN3), or an organic derivative in which the hydrogen atom of hydrazoic acid is replaced by a hydrocarbon group, as in alkyl or aryl azide (RN3), or by an acyl (carboxylic acid) group, like in acyl azide.
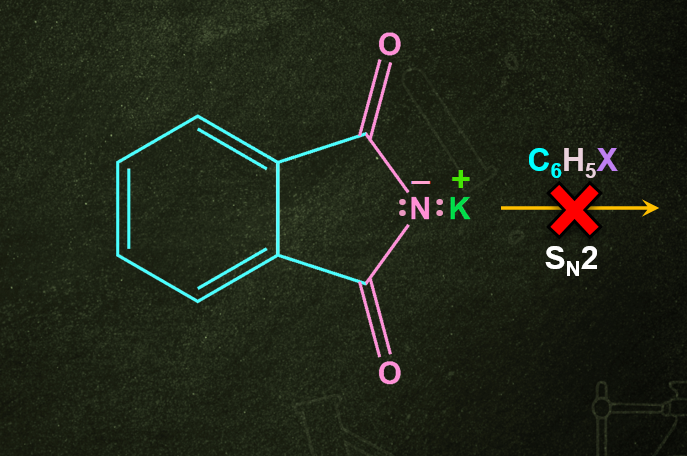
- Gabriel phthalimide synthesis is used for the production of primary amine but during the process of reaction high-temperature condition is needed for the cleavage of phthalimide with hydrazine.
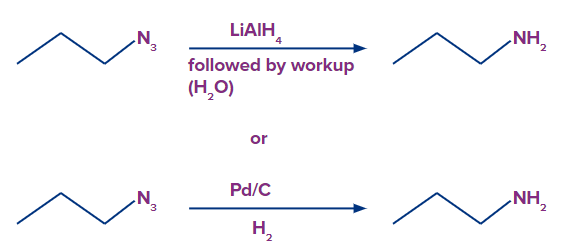
- Note the triple bond between nitrogen and nitrogen in the resonance form above. Organic azides can be converted to primary amines and released N2when handled with a reducing agent like LiAlH4or even catalytic hydrogenation (Pd/C, H2). This provides a very practical pathway from alkyl halides to primary amines.
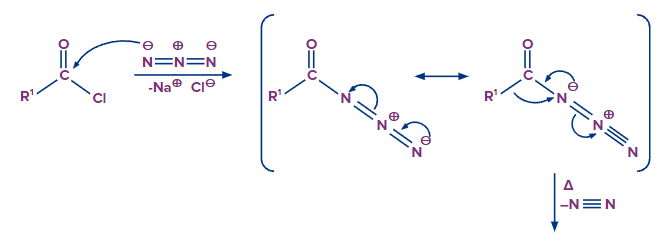
When acyl chloride is treated with sodium azide, acyl azide/acid azide is formed.
Acid azides can be converted to isocyanides when acid azide is heated in presence of nickel. Isocyanides further convert into a primary amine, when isocyanide is dehydrated and a rearrangement reaction follows up.
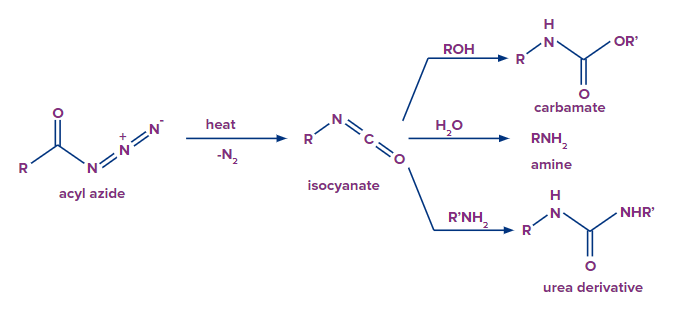
The reaction is commonly is called as curtis rearrangement reaction. The mechanism of this reaction is discussed below.
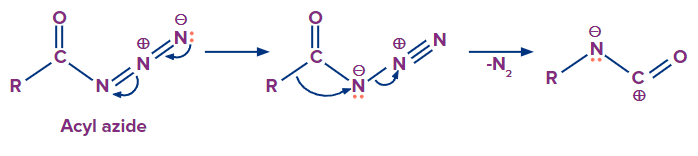
The mechanism of this reaction involves following steps
Formation of Isocyanate
Step -1
Migration of carbon atom to displace the nitrogen gas leaving group on an adjacent nitrogen atom.
Step-2
Formation of the isocyanate by donation of a lone pair of electrons from nitrogen atom to the carbocation
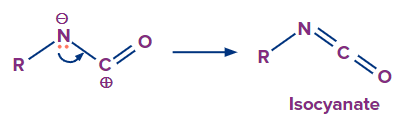
Transformation of isocyanate
Addition of water molecule results in the formation of carbamic acid from isocyanate. Carbamic acid is generally unstable and it quickly loses carbon dioxide to give a primary amine.
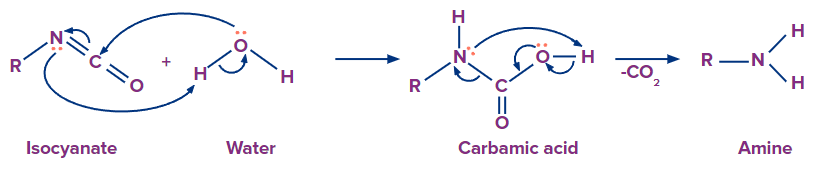
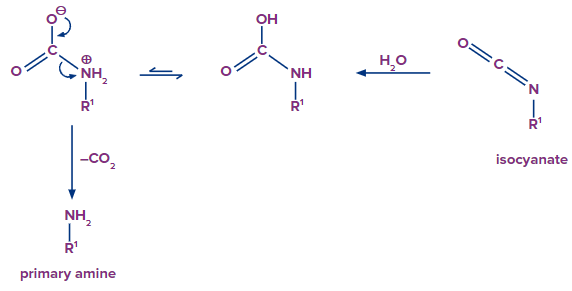
Preparation of amines from the reduction of oximes
Oximes are chemical substances that fall within the imine class and have the general formula R1R2C=N O H. Where R1 is the organic side chain and R2 is hydrogen, creating an aldoxime or a molecule similar to another class of organic compounds like ketoxime. A category of substances that are extremely closely linked is the O-substituted group of oximes.
- Oximes, which are made from hydroxylamine, a ketone, and an aldehyde, are also known as nitrogen-containing organic molecules.
- These compounds can also be created via the isomerization of nitroso compounds or by the reaction of nitro compounds with hydrogen-donating reagents.
- Aldoximes, or oximes made from aldehydes, can also be dehydrated to create nitriles.
- The other chemical reactions include turning it into amides and turning it into amines by using hydrogen or other reducing agents. By reacting it with potent acids, this is produced.
Oxime has a two-sided chain-like structure with a carbon atom at its centre. The two side chains are completely different from one another. A hydroxyl group is present in one of the two chains.
Aldicarb oxime, aldoxime, dimethylglyoxime, ketoxime, methoxime, etc. are a few examples of oximes.
Oxime is created and water molecules are eliminated when an aldehyde or ketone combines with hydroxylamine (NH2OH) in a weakly acidic media.
- When acetaldehyde reacts with hydroxylamine it produces acetaldoxime
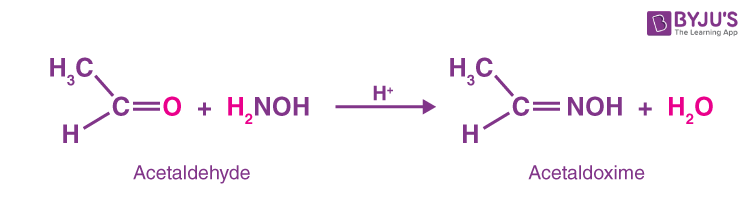
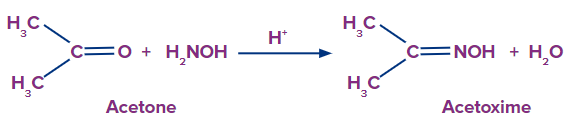
- When acetone reacts with hydroxylamine it produces acetoxime.
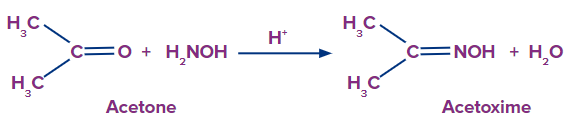
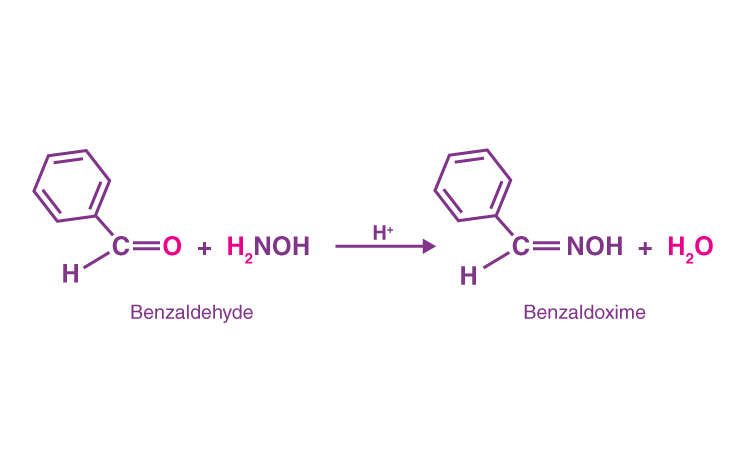
- Hydroxylamine and benzaldehyde combine to generate benzaldoxime and water.
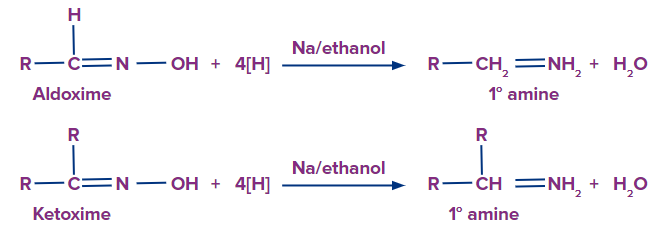
Now the oxime is reduced in presence of a reducing agent to give primary amine
Practice problems
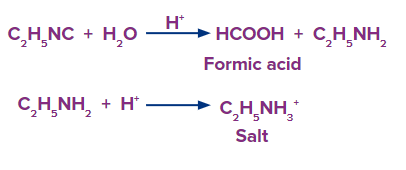
Q1. In an acidic media, ethyl isocyanide hydrolyzes to produce
a. Ethylamine salt and methanoic acid
b. Butanoic acid and ammonium salt
c. Acetic acid and ammonium salt
d. None of the above
Answer: (A)
Hydrolysis refers to cleaving a bond in presence of a water molecule.
During the hydrolysis of ethyl isocyanide in presence of an acidic medium it produces ethylamine and methanoic acid.
Q2. Which of the following is not a reducing agent?
a. Hydrogen peroxide
b. LiAlH4
c. Sn/HCl
d. Fe/HCl
Answer: (A)
Solution: One of the reactants of an oxidation-reduction process is a reducing agent, which decreases the other reactant by discharging electrons to it. The reduction process cannot take place if the reducing agent does not transfer electrons to another component during a reaction. LiAlH4, Sn/HCl & Fe/HCl are some of the examples of reducing agents. Hydrogen peroxide (H2O2) is an oxidizing agent. This reagent reduces itself by oxidizing the reactant.
Q3. When ethyl nitrile is treated with Sn/HCl it produces
a. Ethanol
b. Acetone
c. Acetaldehyde
d. ethylamine
Answer: (D)
Nitriles which are obtained from aldehydes undergo partial reduction to form imine, this reaction is commonly known as Stephen reduction. Imine on further reduction converts into primary amine in the presence of reducing agents that is Sn/HCl. This reaction is used for the preparation of amines containing one carbon atom more than the starting compound. Ethylnitrile gives ethylamine in presence of Sn/HCl.
CH3CN+4[H]CH3CH2NH2
Q4. Acetoxime can be prepared from
a. Quinone
b. Propanone
c. Acetone
d. pivaldehyde
Answer: (C)
When acetone reacts with hydroxylamine it produces acetoxime.
Frequently asked questions (FAQs)
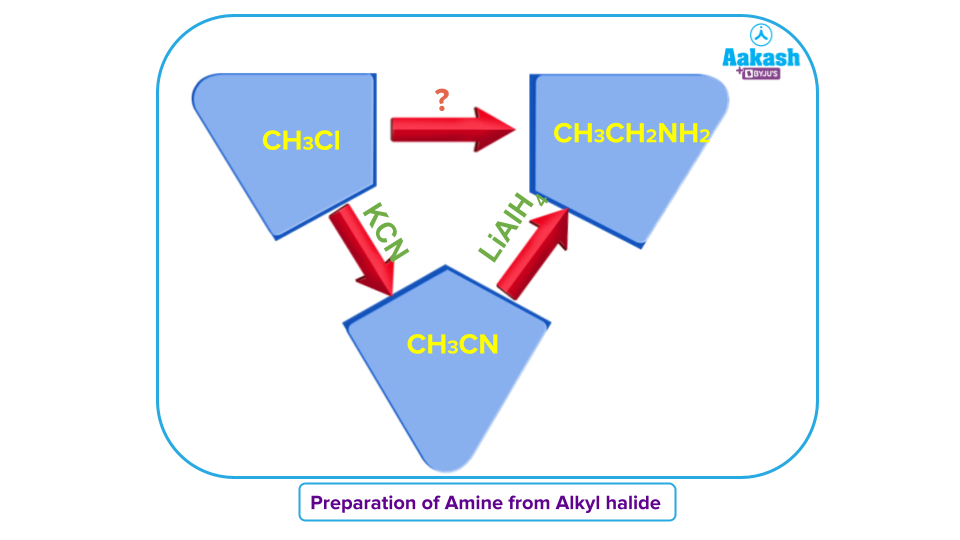
Q1. Can we prepare amine from halides?
Answer: A two-step procedure can be used to transform alkyl halides into primary amines. The alkyl halide is first changed into a nitrile through an SN2reaction with a cyanide anion. LiAlH4then causes the nitrile to be converted to a primary amine . An extra carbon atom is introduced throughout this chain of reactions.
Q2. Can any other functional group be reduced by Na/EtOH reagent?
Answer: Yes, esters can be reduced by Na/EtOH alcohol. This process is commonly know as Bouveault-Blanc reduction. This method could be used to reduce esters, turning them into two moles of alcohol as a result of the usage of sodium metal and absolute ethanol.
Q3. Why does reducing alkyl cyanide result in primary amine?
Answer: Primary amines can be produced by entirely reducing the triple bond between the carbon and nitrogen in nitriles. H2is used for the reduction, using either LIAIH4 or a catalyst of Ni or Pt.
Q4. Can we prepare aromatic amines from Gabriel phthalimide synthesis?
Answer: Gabriel phthalimide synthesis cannot produce aromatic primary amines because aryl halides do not undergo nucleophilic substitution with the salt created by phthalimide.