-
Call Now
1800-102-2727
Redox Process- Redox Process, Example, Types, Applications, Practice Problems and FAQs
Many people have probably witnessed a driver being asked to blow into a handheld device to test their sobriety.
But do you know anything about the device used for the breath test and the science behind it?
The device is known as a breathalyser, and it uses the redox process to quantify the alcohol content.

The potassium dichromate chemical present in the device reacts with ethanol in the breath. Dichromate loses an oxygen atom and gets transformed from orange-coloured dichromate to green-coloured chromium sulphate.
At the same time, ethanol gains an oxygen atom, resulting in the formation of acetaldehyde.
The reactions are said to be reduction and oxidation respectively or simply redox processes as they always occur together.
When both oxidation and reduction processes are involved, the process is known as the Redox process.
Table of Contents
- Redox Process
- Redox Reaction Example
- Types of Redox Process
- Applications of Redox Process
- Practice Problems
- Frequently Asked Questions
Redox Process
A redox reaction is one in which two distinct reactants participate and electrons are transferred between them. The redox process involves two processes through which electrons are transferred. We should have a good knowledge of the following terms in order to understand the redox process in a better way.
Reduction: Any chemical reaction in which an element gains electrons is referred to as a reduction. It actually means that the element whose oxidation number is decreasing has been reduced.
Oxidation: Any chemical reaction in which an element loses electrons is referred to as oxidation. It actually means that the element whose oxidation number is increasing has been oxidised.
Oxidizing agent or Oxidant: During a chemical reaction, an oxidant is a compound that can oxidise others while also reducing itself or a reagent that gains electrons in a redox process.
Reducing agents or Reductants: During a chemical reaction, a reductant is a compound that can reduce others while oxidising itself or a reagent that loses electrons in a redox process.
Redox Reaction Example
A reduction-oxidation or redox process involves two processes one is the oxidation process and the other is the reduction process. The redox process occurs when both reduction and oxidation occur at the same time. The reduced species gains electrons while the oxidised species loses them.
It is possible to pinpoint electron transfer during a reaction by looking for distinctive oxidation state conversions. The graphic that follows demonstrates in great detail how an electron is exchanged between two reactants during a redox process or redox reaction.
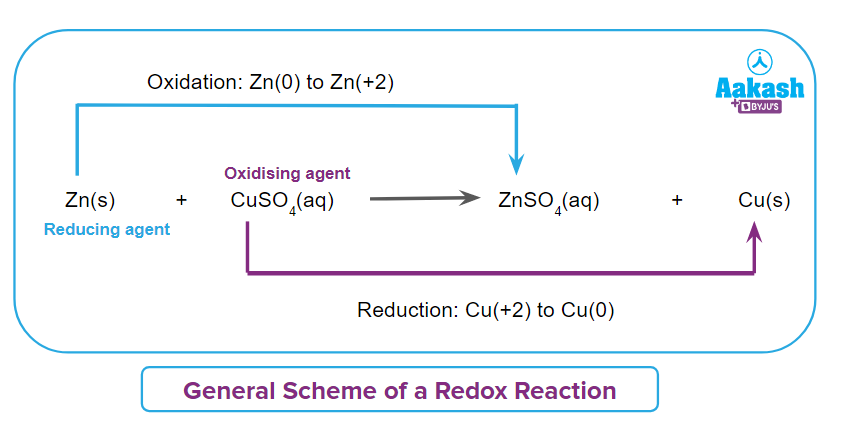
The overall reaction may hence be considered as a combination of two half-reactions. The oxidation half-reaction and the reduction half-reaction are the two parts of every redox process. Each of these half-reactions must balance when written separately in order for the number of electrons given in the oxidation half reaction to be equal to the number of electrons gained in the reduction half reaction.
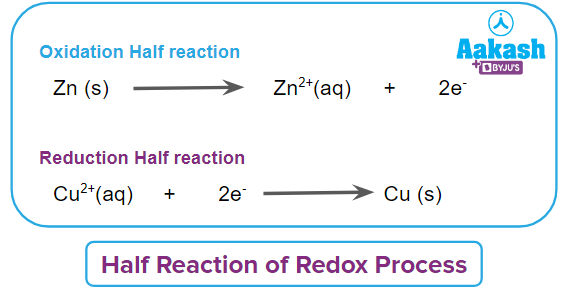
In this redox process or redox reaction, the oxidation number of Zinc in Zn(s) is 0 which changes to +2 in ZnSO4(aq). The oxidation number of Zinc increases by two. It means that there is an increase in the oxidation number of Zinc from (0 to +2).
In this redox process, the oxidation number of Copper in CuSO4(aq) is +2 and changes to 0 in Cu(s). The oxidation number of Copper decreases by two. It means that there is a reduction in the oxidation number of copper from (+2 to 0).
As zinc (Zn(s)) has reduced the copper (CuSO4(aq)) by giving two electrons and itself gets oxidised, Hence Zn(s) acts as reducing agent.
Or we can say copper (CuSO4(aq)) has oxidised the zinc (Zn(s)) by taking two electrons and itself get reduced, Hence CuSO4(aq) acts as oxidising agent.
Types of Redox Process
There are five different types of redox processes or redox reactions present in chemistry. These are
- Decomposition reaction
- Displacement Reaction
- Combination Reaction
- Disproportionation Reaction
- Combustion Reaction
- Decomposition Reaction
When a compound is separated into various compounds, this kind of reaction is known as a decomposition reaction.
Example:
However, all decomposition reactions do not come under the redox process.
For instance,
- Combination Reaction
When various compounds are combined to form a new compound, this kind of reaction is known as a combination reaction. It is the reverse of the decomposition reaction.
Example:
However, all combination reactions do not come under the redox process.
For instance,
- Displacement Reaction
When an atom or an ion from a compound is replaced by an atom or an ion from another compound, this kind of reaction is known as a Displacement Reaction.
It can also be written as
Displacement reactions can be further categorised into two reactions
(i) Metal displacement reaction
When a metal ion or atom from a compound is replaced by metal from another compound, this kind of reaction is known as a Metal displacement Reaction.
(ii) Non-Metal displacement Reaction
When a non-metal ion or atom from a compound is replaced by a non-metal from another compound, this kind of reaction is known as a Metal displacement Reaction.
- Disproportionation Reactions
Disproportionation reactions are those that involve the oxidation and reduction of a single reactant. It means a single compound is going to be oxidised as well as reduced as well.
- Combustion Reactions
A combustion reaction is a type of redox reaction in which a substance reacts with molecular oxygen to form oxygen-containing compounds of other elements in the reaction. Combustion is categorised as an oxidation-reduction reaction, making it a redox reaction.
(g)
Applications of Redox Process
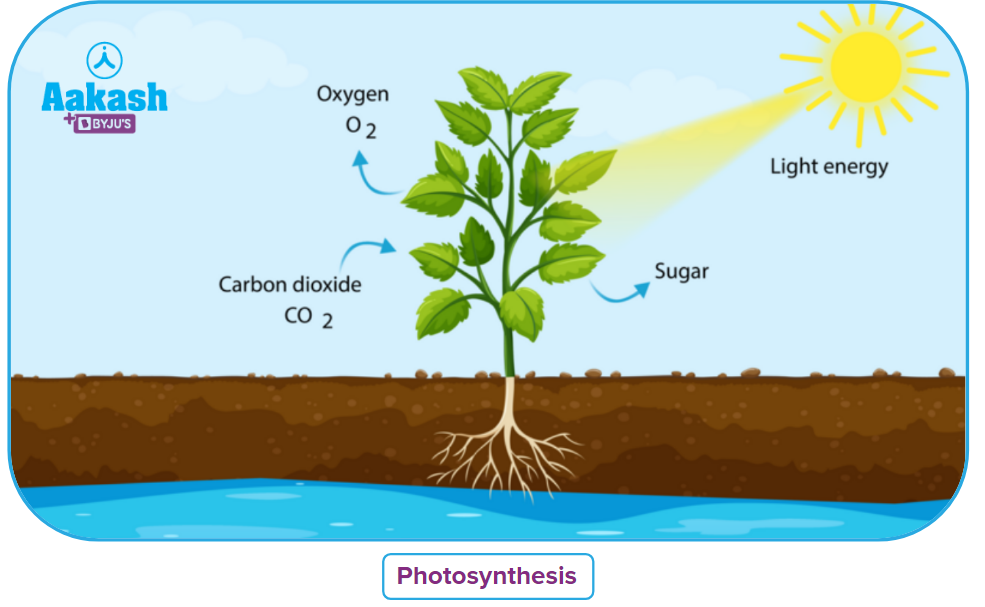
(i) Redox Process in Photosynthesis
The process through which water and carbon dioxide are transformed into carbohydrates in green plants is known as photosynthesis.
The involved reaction is
In this reaction, the oxidation number of C in CO2(g) is +4 and changed to 0 in C6H12O6(aq). The oxidation number decreases which means the carbon is reduced in this reaction and CO2(g) acts as oxidising agents.
In this reaction, the oxidation number of O in H2O(l) is -2 which is changed to 0 in O2(g). The oxidation number increases which mean O is oxidised in this reaction and O2(g) acts as a reducing agent.
As CO2(g) acts as an oxidising agent and O2(g) acts as reducing agent. Hence, this is a redox process which involves both oxidation and reduction.
(ii) Redox Process in Inverters
Let us take an example of Storage batteries that are frequently used in motors and inverters. It comprises a grid of lead packed with lead dioxide (PbO2) as the cathode and a lead anode. The electrolyte is a 38% solution of sulfuric acid.

The reaction can be given as
In this reaction, the oxidation number of Pb in PbO2(s) is +4 and changed to +2 in PbSO4 (s) . The oxidation number decreased which means the lead is reduced in this reaction and PbO2(g) acts as oxidising agents.
In this reaction, the oxidation number of Pb in Pb(s) is 0 that changes to +2 in PbSO4 (s) . The oxidation number increases which mean Pb is oxidised in this reaction and Pb(s) acts as a reducing agent.
As PbO2(g) acts as an oxidising agent and Pb(s) acts as a reducing agent. Hence, this is a redox process which involves both oxidation and reduction.
Practice Problems
Q1. Which of the following reactions is the redox process?
Answer: B)
Solution: A chemical reaction in which both the process; oxidation and reduction process are included, they are referred as an oxidation-reduction process or redox process.
Option A shows that no element's oxidation state changes, indicating that no oxidation or reduction is occurring. This means the process is not redox in nature.
remains the same after the process.
Option B shows that no element's oxidation state changes, indicating that no oxidation or reduction is occurring. This means the process is not redox in nature.
remains the same after the reaction.
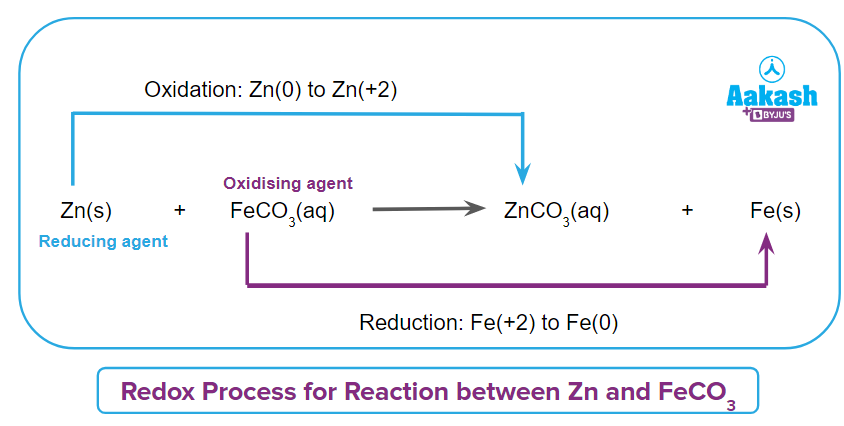
Option C clearly shows a change in the oxidation states of Zn and Fe, which involves oxidation and reduction at the same time. As a result, the given reaction is a redox reaction.
Option D shows that no element's oxidation state changes, indicating that no oxidation or reduction is occurring. This means the process is not redox in nature.
that remains the same after the reaction.
Q2. Which of the following exists as a reducing agent?
- SO3
- H2S
- CaSO4
- None of these
Answer: B)
Solution:
In options (A) and (C),
Oxidation state of S in CaSO4 = x
Oxidation state of S in SO3 = y
S is in the highest oxidation state (+6). So, S can accept electrons only and exist in lower oxidation states (+4, +2, -2). Hence, it behaves as an oxidising agent.
In option (B),
the oxidation state of S in H2S = z
+2+z=0
z=-2
S in H2S is in the lowest oxidation state (-2). So, S can donate electrons only and exist in higher oxidation states (+4, +2, +6). Hence, it behaves as a reducing agent.
So, option B) is the correct answer.
Q3. Which of the following element is oxidised and reduced respectively in the given reaction?
- Fe and Cl
- Fe and H
- H and Cl
- H and Fe
Answer: (D)
Solution: Let us find out oxidation states first of all to find out oxidised and reduced substance.
The oxidation state of Fe in FeCl3 = x
x+3(-1)=0
x=+3
Oxidation state of Fe in FeCl2 = y
y+2(-1)=0
y=+2
Oxidation state of H in HCl = a
a-1=0
a=+1
Oxidation state of H in H2 = b
2b=0
b=0
The oxidation state of Chlorine remains same in reactant and product as well. Hence, It will neither oxidised nor reduced. It implies that option (C) can not be a correct answer.
The oxidation state of Fe changes from +3 to +2, a decrease in oxidation number implies clearly that Fe is reduced.
Oxidation state of H changes from 0 to +1, an increase in oxidation number implies clearly that H is oxidised.
As H is oxidised and Fe is reduced, the correct answer is an option (D).
Q4. Select the non-redox process from the given reactions.?
- None of these
Answer: D)
Solution:
There is no oxidation or reduction taking place in process (A). This means the process is not redox in nature.
The oxidation states of the elements [ ] remain the same after the reaction.
There is clearly a change in the oxidation states of H and Zn in reaction (B), which involves both oxidation and reduction. As a result, the reaction in question is a redox reaction.
There is clearly a change in the oxidation states of P in reaction (C), which involves both oxidation and reduction. As a result, the reaction in question is a redox reaction.
Hence, the correct answer is an option (A).
Frequently Asked Questions
Q1. What are some applications of the redox process which we use in our daily life?
Answer: Redox process is used to create gold-plated jewellery. Oxidation-reduction processes are also used to sanitise water and bleach materials. Many chemicals, including chlorine and caustic soda, are produced using redox reactions.
Q2. Is cellular respiration an oxidation or reduction process?
Answer: Redox process occurs during cellular respiration. Respiration is a group of metabolic reactions involving both electron loss and gain. As a result, it is referred to as an oxidation-reduction process or redox process.
Q3. Is apple rotting caused by oxidation?
Answer: When you cut an apple, the polyphenol oxidase (PPO) enzymes in the chloroplasts quickly oxidise the phenolic compounds found naturally in apple tissues, which are colourless precursors to brown secondary products. So, yes, the apple rotting is an oxidation process.
Q4. Why is understanding the redox process important?
Answer: Redox processes take us down an important conceptual route in chemistry. Our understanding of redox progresses from oxygen gain and loss to hydrogen gain and loss. This opens the door to viewing all chemical reactions as electron rearrangements.


